In this issue of Blood Advances, Palacios-Berraquero et al1 described their elegant work combining clinical observations from patient cohorts and ex vivo model systems to elucidate the risk factors and causes of long-term cytopenias in patients with relapsed and refractory multiple myeloma (RRMM) receiving chimeric antigen receptor (CAR) T cells targeting the B-cell maturation antigen (BCMA).
CAR T cells targeting BCMA have produced remarkably high remission rates in patients with RRMM leading to their widespread use.2,3 Further clinical trials are underway to evaluate their efficacy in earlier lines of therapy, including newly diagnosed MM and high-risk smoldering MM. However, a common complication of these therapies is prolonged cytopenias, which occur or persist beyond the first 30 days after CAR T-cell infusion. These long-lasting or late-occurring cytopenias often require the ongoing use of growth factors and place patients at high risk for serious complications related to infections and bleeding. Multiple studies have evaluated the frequency and developed clinical prediction models for these late cytopenias. The most widely used is the CAR-HEMATOTOX model, which was first developed for anti-CD19 CAR T cells and subsequently evaluated in anti-BCMA CAR T cells.4,5 Validated risk factors for long-term cytopenias include low blood counts at baseline as well as increased levels of inflammatory markers (eg, C-reactive protein and ferritin).
This study by Palacios-Berraquero et al adds to the literature by studying a cohort of 48 patients with RRMM who underwent treatment with anti-BCMA CAR T cells. Long-term cytopenias in this cohort were associated with baseline cytopenias, which suggests that disease-associated factors and toxicity from prior treatment may be contributors. In addition, both baseline and peak inflammatory markers such as ferritin were associated with an increased risk of cytopenias. Persistent elevation of the ferritin was seen in patients with the longest-lasting cytopenias, suggesting that sustained inflammation initiated by CAR T cells may provide ongoing suppression of normal hematopoiesis. Although establishing the clinical risk factors for cytopenias is important, a more complete understanding of the mechanisms that lead to prolonged cytopenias is needed to inform preventive and treatment strategies.
Cytopenias in patients with MM receiving CAR T cells may be caused by multiple mechanisms. Patients with RRMM often have cytopenias before undergoing CAR T-cell treatment related to their underlying disease (anemia is a hallmark feature of MM) and prior therapy. Rates of clonal hematopoiesis and clonal cytopenia of undetermined significance are higher in patients undergoing CAR T-cell treatment than in age-matched individuals.6,7 In addition, therapy-related myeloid neoplasms (t-MNs) have been frequently reported in patients after CAR T-cell treatment, suggesting that the development and evolution of myeloid malignancy may contribute to long-lasting cytopenias in some patients.3 The presence of t-MNs should be ruled out in any patient undergoing evaluation for persistent or worsening cytopenias.
All patients receiving CAR T cells also receive conditioning chemotherapy, typically a combination of fludarabine and cyclophosphamide. Although conditioning regimens deplete lymphocytes and create an environment conducive to CAR T-cell expansion, they also suppress hematopoiesis. After lymphodepletion, patients typically begin to recover their blood counts after several days at a time that often coincides with the peak expansion of the infused CAR T-cell product. Blood counts often then drop again in this biphasic pattern, which is believed to be caused by inflammatory cytokines elaborated by the rapidly expanding and activated T cells. In some cases, this process can develop into a fatal hyperinflammatory syndrome akin to hemophagocytic lymphohistiocytosis, known as immune effector cell hemophagocytic syndrome (IEC-HS), which is believed to be driven by high levels of interferon gamma (IFN-γ)–mediated signaling.8,9 Whether all cases of long-term cytopenias are caused by an inflammatory process that lies within the IEC-HS spectrum or a combination of all the processes described above is not well understood.
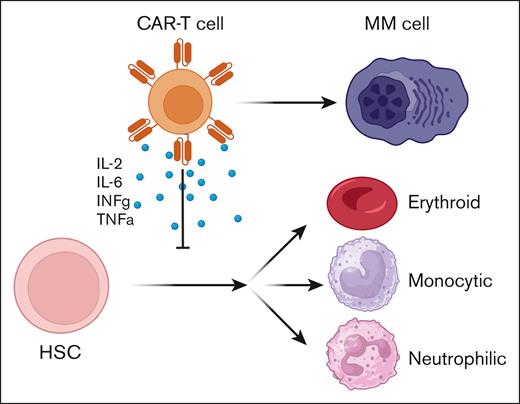
Both acute and chronic inflammation suppress normal hematopoiesis through a variety of mechanisms.10 However, how the specific inflammation driven by CAR T-cell expansion and activity affects hematopoiesis is not well characterized. In addition, we do not have a clear understanding of how the specific cytokines elaborated by activated anti-BCMA CAR T cells affect normal hematopoiesis and how this process could be pharmacologically interrupted. To study this process in more detail, Palacios-Berraquero et al developed a hematopoietic differentiation assay in which human CD34+ hematopoietic stem and progenitor cells (HSPCs) were exposed ex vivo to supernatants collected from activated CAR T cells and differentiation was measured. This inflammatory supernatant contained high levels of many cytokines known to be produced during CAR T-cell expansion, including interleukin-2 (IL-2), IL-6, IFN-γ, and tumor necrosis factor α (TNF-α). HSPCs exposed to this inflammatory milieu displayed impaired production of neutrophilic, monocytic, and erythroid precursors, a phenotype that was reversed upon the addition of a mixture of inhibitors of IFN-γ, TNF-α, transforming growth factor β, IL-6, and IL-17 (see figure). However, it remains unclear whether this effect is mediated predominantly by only 1 or 2 of these inflammatory cytokines, or all of them together. Single-cell RNA sequencing demonstrated that this phenotype was likely mediated by the activation of pathways that lead to differentiation arrest and apoptosis. Computational analysis to identify complex gene regulatory networks demonstrated that genes involved in mediating signaling by IL-17, IL-18, and the TNF superfamily were highly upregulated in HSPCs. Further work dissecting the role of individual cytokines in hematopoietic differentiation will provide important preclinical data to guide future efforts to pharmacologically target these pathways to prevent or treat cytopenias in our patients. Integrating observations from clinical cohorts with these preclinical models will allow us to identify novel therapeutic approaches to more safely administer these highly effective cellular therapies to all patients who need them.
Schematic demonstration of how activation of CAR T cells by contact with MM cells leads to the elaboration of inflammatory cytokines that suppress hematopoietic stem cell (HSC) differentiation into mature blood cells.
Schematic demonstration of how activation of CAR T cells by contact with MM cells leads to the elaboration of inflammatory cytokines that suppress hematopoietic stem cell (HSC) differentiation into mature blood cells.
Conflict-of-interest disclosure: A.S.S. has received consulting fees from Roche and Novartis.