Key Points
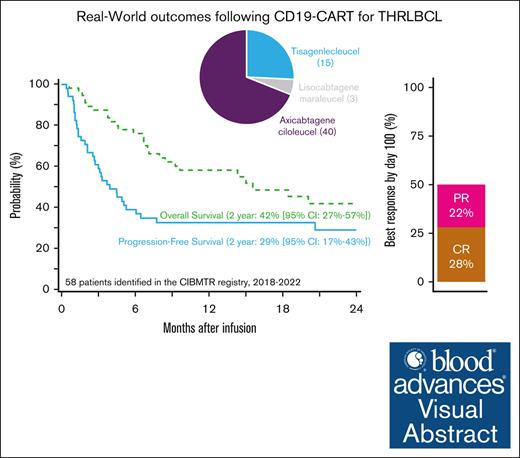
The 2-year progression-free survival of 58 patients with T-cell/histiocyte–rich large B-cell lymphoma treated with CD19-CART therapy was 29%.
Visual Abstract
T-cell/histiocyte–rich large B-cell lymphoma (THRLBCL) is a rare histologic variant of LBCL. Limited data regarding CD19–directed chimeric antigen receptor T-cell (CART) therapy in relapsed/refractory (R/R) THRLBCL suggest poor efficacy. We investigated CART outcomes for R/R THRLBCL through the Center for International Blood and Marrow Transplant Research registry. A total of 58 adult patients with R/R THRLBCL who received commercial CD19-CART therapy between 2018 and 2022 were identified. Most patients (67%) had early relapse of disease (45% primary refractory) with a median of 3 (range, 1-7) prior therapies and were treated with axicabtagene ciloleucel (69%). At median follow-up of 23 months after CART therapy, 2-year overall and progression-free survival were 42% (95% confidence interval [CI], 27-57) and 29% (95% CI, 17-43), respectively. In univariable analysis, poor performance status before CART therapy was associated with higher mortality (hazard ratio, 2.35; 95%CI, 1.02-5.5). The 2-year cumulative incidences of relapse/progression and nonrelapse mortality were 69% and 2%, respectively. Grade ≥3 cytokine release syndrome and immune effector cell–associated neurologic syndrome occurred in 7% and 15% of patients, respectively. In this largest analysis of CD19-CART therapy for R/R THRLBCL, ∼30% of patients were alive and progression free 2 years after CART therapy. Despite a high incidence of progression (69% at 2 years), these results suggest a subset of patients with R/R THRLBCL may have durable responses with CARTs.
Introduction
T-cell/histiocyte–rich large B-cell lymphoma (THRLBCL) is a rare histologic variant that comprises <10% of LBCL and is classified as a separate entity based on the morphologically distinct appearance.1 The fifth edition of World Health Organization classification considers THRLBCL at the extreme end of a spectrum of growth patterns of nodular lymphocyte–predominant Hodgkin lymphoma (NLPHL) with a more aggressive clinical behavior.2 NLPHL and THRLBCL also share highly recurrent genetic lesions supporting a close relationship3,4 and distinguishing between these entities can be challenging. Because of limited available evidence regarding subtype-specific outcomes in patients with THRLBCL, they are managed like diffuse LBCL (DLBCL) in the frontline and relapsed settings.
A study of the tumor microenvironment (TME) in THRLBCL biopsy samples identified Programmed cell death protein-1 (PD-1)/Programmed cell death ligand-1 (PD-L1) signaling as a possible pathogenic mechanism and driver of immune escape.5 Although retrospective studies in the rituximab era have reported survival rates comparable with DLBCL with intensive chemoimmunotherapy,6,7 the treatment of relapsed disease remains an unmet need because of poor outcomes.8 Although CD19–directed chimeric antigen receptor T-cell (CART) therapy has shown efficacy in relapsed/refractory (R/R) DLBCL,9-12 its role in THRLBCL remains largely undefined. In published case series of patients with R/R THRLBCL, almost all patients experienced early treatment failure after CART infusion.8,13 These reports have brought into question the role of anti-CD19 CART therapy in R/R THRLBCL, especially as we have learned more about its unique TME. Therefore, we investigated the outcomes of patients with R/R THRLBCL treated with US Food and Drug Administration–approved commercial CARTs in the real world through the Center for International Blood and Marrow Transplant Research (CIBMTR) registry.
Methods
Data source
The CIBMTR is a working group comprising >380 transplantation centers worldwide that provide data regarding cellular therapies to a statistical center at the Medical College of Wisconsin. Data quality is augmented through computerized affirmation of discrepancies, physicians’ review of submitted data, and on-site audits of participating centers. Observational studies are conducted by the CIBMTR in compliance with all pertinent federal regulations with regard to protection of human research participants. All patients included in this analysis have provided written consent for research. The institutional review board of Medical College of Wisconsin has approved this study. Patient provide informed consent for their data to be reported to the CIBMTR.
Patients
Adult patients (aged ≥18 years) with THRLBCL who received US Food and Drug Administration–approved commercial CD19-directed CART products (axicabtagene ciloleucel [axi-cel], tisagenlecleucel [tisa-cel], or lisocabtagene maraleucel [liso-cel]) as their first cell therapy infusion between 2018 and 2022 were identified in the CIBMTR registry. Patients were excluded if they had received prior adoptive cellular therapy, not consented for research, or treated at embargoed or European Union centers.
Definitions and end points
Overall survival (OS) was the primary outcome. Patients alive without evidence of disease relapse or progression were censored at last follow-up. Death from any cause was considered an event for OS analysis. Secondary outcomes included progression-free survival (PFS), cumulative incidence of progression/relapse (CIP/R), nonrelapse mortality (NRM), incidence of cytokine release syndrome (CRS), and immune effector cell–associated neurologic syndrome (ICANS). For PFS, progression/relapse or death from any cause were considered events. NRM was defined as death without evidence of prior lymphoma progression/relapse; relapse was considered a competing risk. CIP/R was defined as relapsed lymphoma after CART therapy; NRM was considered a competing risk. Bridging was defined as any therapy, including radiation, administered between apheresis and CART infusion, or patients’ last line of treatment before CARTs if it was continued after apheresis. Disease response to last line of therapy at the time before CART therapy was defined using the Lugano classification.14,15
Statistical analysis
Baseline characteristics of the study population were described. CRS and ICANS were reported according to the consensus American Society for Transplantation and Cellular Therapy criteria.16 Kaplan-Meier estimates were used for OS and PFS. Forest plots were created to present hazard ratios and their 95% confidence intervals (CIs) based on the univariable Cox model. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
A total of 58 patients from 37 centers met the study inclusion criteria and were included in the analysis. Baseline patient and disease characteristics are summarized in Table 1. Before CART therapy, 17 (29%) patients had bulky disease (>5 cm), and 12 (21%) had bone marrow involvement. The median age at CART infusion was 49 years (range, 19-76), with 12 patients (21%) were aged >65 years. Patients were predominately male (79%), white (69%), and non-Hispanic/non-Latino (88%). One-third of patients (n = 19) had a comorbidity score17 of ≥3, and 24 (41%) had a Karnofsky performance status of <90. Most patients (n = 39, 67%) had early relapse of THRLBCL (within 12 months of first-line therapy), including 26 (45%) with primary refractory disease. The median number of prior therapies was 3 (range, 1-7), which included 21 (36%) patients with prior autologous stem cell transplantation and 1 (2%) patient with prior allogeneic stem cell transplantation.
Axi-cel was the predominant CART product (n = 40, 69%), followed by tisa-cel (n = 15, 26%) and liso-cel (n = 3, 5%). Bridging therapy was reported in 18 (31%) patients, most commonly multi- or single-agent chemotherapy (23%). Almost all patients (93%) had active disease before CART therapy; only 4 patients were in complete remission (CR).
CRS was reported to occur in 40 (69%) patients: 26 (45%) grade 1, 10 (17%) grade 2, 2 (3%) grade 3, and 1 (1.7%) each grade 4 and 5. ICANS was reported in 17 (29%) patients: 5 (9%) grade 1, 3 (5%) grade 2, 4 (7%) grade 3, and 5 (9%) grade 4 (supplemental Table 1). By day 100 after CART infusion, best overall response rate (ORR) was 50%, with 28% CR and 22% partial responses. ORR and CR rates were similar between CART products (supplemental Table 2).
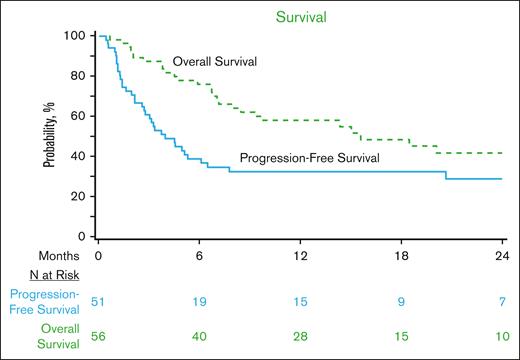
With a median follow-up of 23 months (range, 2-48) from CART infusion, 2-year OS was 42% (95% CI, 27-57), and PFS was 29% (95% CI, 17-43; Figure 1). Among patients who achieved a CR as of day 100, the 2-year cumulative incidence of relapse was 33% (95% CI, 5-71), corresponding to a 2-year OS and PFS among these patients of 92% (95% CI, 73-100) and 67% (95% CI, 32-94), respectively. Considering only those patients who were not already in CR at infusion (n = 54), the 2-year OS and PFS were 39% (95% CI, 24-56) and 25% (95% CI, 12-40), respectively.
OS and PFS of patients with relapsed THRLBCL treated with CD19-CART therapy in the CIBMTR registry.
OS and PFS of patients with relapsed THRLBCL treated with CD19-CART therapy in the CIBMTR registry.
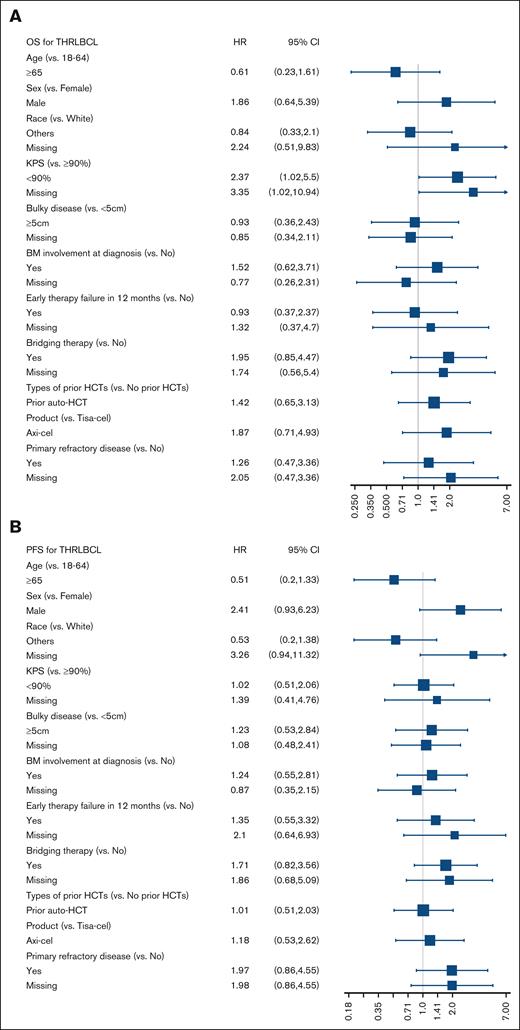
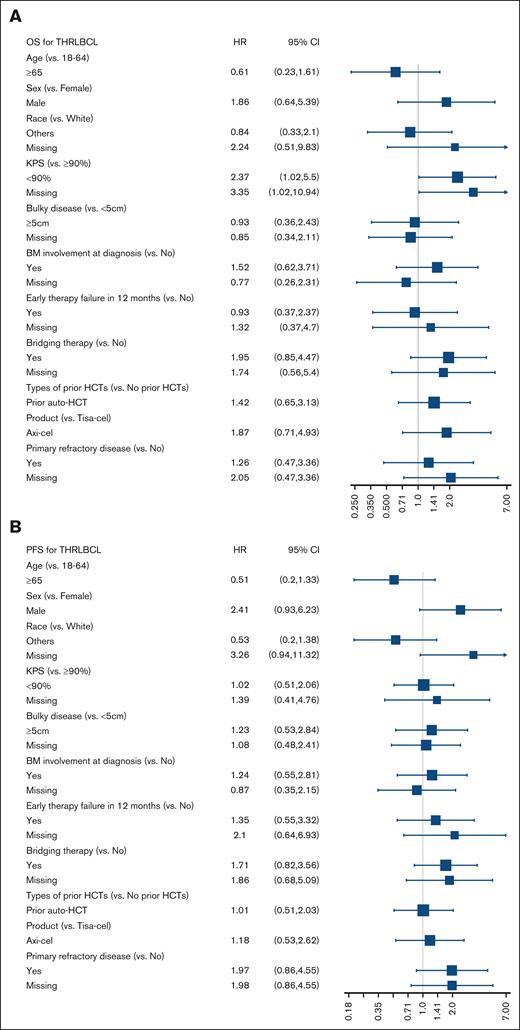
In a univariable analysis, there were no significant associations with PFS or OS except Karnofsky performance status of <90 before CART infusion, which was associated with significantly higher risk for mortality hazard ratio of 2.37 (95% CI, 1.02-5.5; Figure 2). The 2-year CIP/R was 69% (95% CI, 55-82) and NRM was 2% (95% CI, 0-8). A total of 30 deaths were reported during the follow-up period, with lymphoma recurrence/progression, seen in 23 (77%) patients, being the most common cause of death (supplemental Table 3).
Forrest plots of factors associated with survival outcomes. Univariable analysis of association with (A) OS and (B) PFS. allo-HCT, allogeneic hematopoietic cell transplantation; auto-HCT, autologous hematopoietic cell transplantation; KPS, Karnofsky performance status.
Forrest plots of factors associated with survival outcomes. Univariable analysis of association with (A) OS and (B) PFS. allo-HCT, allogeneic hematopoietic cell transplantation; auto-HCT, autologous hematopoietic cell transplantation; KPS, Karnofsky performance status.
Discussion
THRLBCL is a rare aggressive histologic subtype of LBCL. The pivotal clinical trials that led to the approval of CART products (axi-cel, tisa-cel, and liso-cel) predominantly enrolled patients with the DLBCL, not-otherwise–specified histology.11,12,18 CD19-directed CARTs can be used for treating patients with R/R THRLBCL; however, its therapeutic efficacy is not well established. In this largest study to date of R/R THRLBCL treated with CART therapy, we found that nearly 30% of patients were alive and progression free 2 years after CART. The day-100 post-CART ORR and CR rates were 50% and 28%, respectively. Prior real-world analysis from the CIBMTR of patients treated with axi-cel19 and tisa-cel20 for R/R LBCL have reported higher day-100 ORR of 60% to 73% and CR rates of 44% 56% but similar 2-year PFS of 28% to 36% and OS of 44% to 45%. Thus, post-CART outcomes of patients with R/R THRLBCL overall appear potentially less favorable than DLBCL but some patients do experience durable responses.
As our understanding of the TME of THRLBCL increases, future therapeutic options may emerge. Griffin et al found that malignant THRLBCL B-cells can have PD-L1/PD-L2 copy gain or amplification in 64% of cases associated with increased PD-L1 expression. Their study also reported clinical responses to PD-1 blockade in 3 of 5 patients with R/R THRLBCL, including 2 CR and 1 partial responses.5 A multicenter case series by Trujillo et al reported that all 9 patients with THRLBCL had progressive disease by day 90 after CART infusion.13 They noted evidence of adequate CART expansion in all 3 cases studied, and CD19 expression remained intact in all 5 assessable cases on progression after CART. The authors observed high coexpression of PD-1 and observed objective responses in 2 of 5 patients treated with anti–PD-1 therapy after CART progression. Thus, they hypothesized that THRLBCL is inherently CART resistant because of its unique TME. Checkpoint inhibitor therapy with anti–PD-1 antibodies is uncommonly used for patients with relapsed aggressive LBCL because of low efficacy, including in the post-CART setting.21,22 However, it is possible that in certain histologies such as THRLBCL in which there is a biological rationale, combining PD-1 blockade with CD19 CARTs may be a reasonable approach to try and overcome CART resistance. Trials are currently underway to investigate this strategy (ClinicalTrials.gov identifier: NCT05934448).
Our study has limitations inherent to real-world studies, such as lack of central confirmation of pathologic diagnosis (including history of NLPHL), missing details on disease burden (eg, lactate dehydrogenase) as well as bridging and subsequent therapies after CART progression, day-30 post-CART response, duration of response, and loss to follow-up. However, this is the largest cohort of R/R THRLBCL treated with CART therapy with 93% and 86% patients having 1- and 2-year post-CART follow-up, respectively.
In summary, our study found that ∼30% of patients with R/R THRLBCL may have durable responses with CARTs, indicating that CD19-directed CART therapy remains a potentially curative therapeutic option for this rare disease. However, the risk of progression remains high. Current research is evaluating combination strategies with anti–PD-1 antibodies to improve outcomes for patients with THRLBCL.
Acknowledgments
The Center for International Blood and Marrow Transplant Research is supported primarily by Public Health Service U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung, and Blood Institute, and the National Institute of Allergy and Infectious Diseases; HHSH250201700006C from the Health Resources and Services Administration; and N00014-20-1-2705 and N00014-20-1-2832 from the Office of Naval Research. Support is also provided by Be The Match Foundation, the Medical College of Wisconsin, the National Marrow Donor Program, and from the following commercial entities: Actinium Pharmaceuticals, Inc; Adienne SA; AlloVir, Inc; Amgen, Inc; Angiocrine Bioscience; Astellas Pharma US; bluebird bio, Inc; Bristol Myers Squibb Co; Celgene Corp; CSL Behring; CytoSen Therapeutics, Inc; Daiichi Sankyo Co, Ltd; ExCellThera; Fate Therapeutics; Gamida Cell, Ltd; Genentech Inc; Incyte Corporation; Janssen/Johnson & Johnson; Jazz Pharmaceuticals, Inc; Kiadis Pharma; Kite, a Gilead Company; Kyowa Kirin; Legend Biotech; Magenta Therapeutics; Merck Sharp & Dohme Corp; Millennium, the Takeda Oncology Co; Miltenyi Biotec, Inc; Novartis Pharmaceuticals Corporation; Omeros Corporation; Oncoimmune, Inc; Orca Biosystems, Inc; Pfizer, Inc; Pharmacyclics, LLC; Sanofi Genzyme; Stemcyte; Takeda Pharma; Vor Biopharma; Xenikos BV. R.S. is supported, in part, through the National Institutes of Health (NIH)/NCI Cancer Center Support Grant (P30 CA008748) and an NIH-NCI K-award (K08CA282987).
Authorship
Contribution: P.A.P., J.A.F., R.S., M.S., A.H., and M.H. conceptualized and designed the study; M.A.-J. and M.H. collected and assembled the data; M.H., M.A.-J., and K.W.A. analyzed the data; all authors contributed to data interpretation; P.A.P prepared the first draft of the manuscript; and all authors revised the manuscript and approved the manuscript.
Conflict-of-interest disclosure: M.H. reports research support/funding from Takeda Pharmaceutical, ADC Therapeutics, Spectrum Pharmaceuticals, and Astellas Pharma; reports consultancy with ADC Therapeutics, Omeros, CRISPR, Bristol Myers Squibb (BMS), Kite, AbbVie, Caribou, Genmab, and Autolus; serves on the speakers bureau of ADC Therapeutics, AstraZeneca, BeiGene, Kite. Data Monitoring Committee: Inc, Genentech, Myeloid Therapeutics, and CRISPR. C.S. has served as a paid consultant for Kite/a Gilead Company, Celgene/BMS, Gamida Cell, Karyopharm Therapeutics, Ono Pharmaceuticals, MorphoSys, CSL Behring, Syncopation Life Sciences, CRISPR Therapeutics, Ipsen Biopharmaceuticals Inc, and GlaxoSmithKline; and has received research funds for clinical trials from Juno Therapeutics, Celgene/BMS, BMS, Precision Biosciences, Actinium Pharmaceuticals, Sanofi-Genzyme, Cargo Therapeutics, and Nkarta. M.S. reports consulting for advisory boards, steering committees, or data safety monitoring committees of AbbVie, Genentech, AstraZeneca, Janssen, BeiGene, BMS, MorphoSys/Incyte, Kite Pharma, Eli Lilly, Mustang Bio, Fate therapeutics, Nurix, and Merck; received research funding from Mustang Bio, Genentech, AbbVie, BeiGene, AstraZeneca, Genmab, MorphoSys/Incyte, and Vincerx; holds stock options in Koi Biotherapeutics; and reports employment with BMS (spouse). J.M. reports grants or contracts from Gilead, Atara, CRISPR Therapeutics, Precision Biosciences, and Scripps Research Institute. T.H. reports consulting fees from BeiGene. P.A.P. reports consulting fees from Seagen. N.G. reports consulting fees from Kite, BMS, Seagen, ADC Therapeutics, and Caribou Biosciences; and reports participation on data safety monitoring board or advisory board for Novartis. L.S. reports serving on a data safety monitoring board for an investigator initiated trial (IIT) at Oregon Health & Science University Myeloma group without associated payments. F.T.A. reports consulting fees from Genentech, AstraZeneca, AbbVie, Janssen, Pharmacyclics, Gilead sciences, Kite pharma, Celgene, Karyopharm, MEI Pharma, Verastem, Incyte, BeiGene, Johnson and Johnson, Dava Oncology, BMS, Merck, Cardinal Health, ADC Therapeutics Therapeutics, Loxo Oncology, Adaptive Biotechnologies, Genmab, Epizyme, Caribou Biosciences, and Cellectar Biosciences; and has participated on a data safety monitoring board or advisory board for Ascentage Pharma and AstraZeneca. D.M. reports consulting fees from AstraZeneca (self and spouse); honoraria from BeiGene; and participation in data safety monitoring board or advisory board for Genmab, ADC Therapeutics, Seagen, and Genentech (spouse). N.A. reports consulting fees and travel support from Kite/Gilead; and honoraria from Nebraska Medical Oncology. The remaining authors declare no competing financial interests.
Correspondence: Mehdi Hamadani, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, 9200 W. Wisconsin Ave, Suite C5500, Milwaukee, WI 53226; email: mhamadani@mcw.edu.
References
Author notes
P.A.P. and J.A.F. contributed equally and are joint first authors.
The Center for International Blood and Marrow Transplant Research (CIBMTR) supports accessibility of research in accordance with the National Institutes of Health data sharing policy and the National Cancer Institute Cancer Moonshot public access and data sharing policy. The CIBMTR only releases deidentified data sets that comply with all relevant global regulations regarding privacy and confidentiality.
The full-text version of this article contains a data supplement.