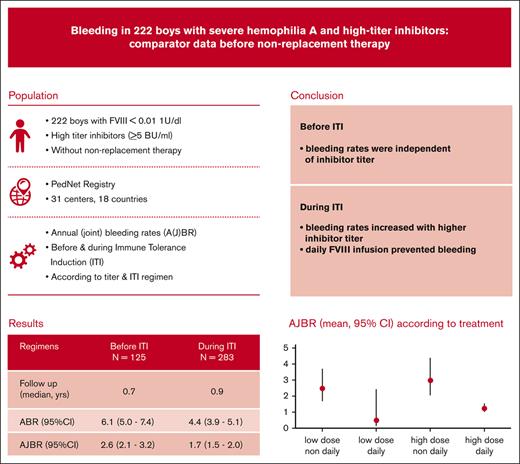
In 222 boys with severe hemophilia A and inhibitors of >5 BU, bleeding was reduced from 6.1 to 4.4 per year during ITI.
Before ITI, bleeding was independent of inhibitor titer; during ITI, bleeding increased with higher inhibitor titer and nondaily ITI.
Visual Abstract
Prevention of bleeding and its consequences is the main goal of hemophilia treatment and determines treatment choices for patients who develop inhibitors. To assess bleeding before and during immune tolerance induction (ITI) and its association with ITI regimen and inhibitor titer, we selected and analyzed data on patients receiving high-titer inhibitors from the international prospective PedNet cohort study. In total, 222 patients with severe hemophilia A and inhibitor titers of >5 Bethesda units (BU) were followed from the first positive to the first negative inhibitor result (median overall follow-up, 1.7 years). Mean annual (joint) bleeding rates (AJBR) and 95% confidence intervals (CIs) were compared according to treatment and inhibitor titer using multivariable negative binomial regression. Before ITI, 115 patients showed an ABR of 6.1 (5.0-7.4) and an AJBR 2.6 (2.1-3.2). Bleeding was independent of inhibitor titer. During ITI, 202 patients had an ABR of 4.4 (3.9-5.1) and an AJBR of 1.7 (1.5-2.0). AJBR during ITI increased with inhibitor titer (hazard ratio [HR] for ≥200 BU vs 5 to 39 BU [4.9; CI, 3.2-7.4]) and decreased with daily ITI infusions (HR, 0.4; CI, 0.3-0.6) or activated prothrombin complex concentrate prophylaxis (HR, 0.4; CI, 0.2-0.8), whereas ITI dose and recombinant activated factor VII prophylaxis did not independently affect bleeding. These data provide evidence for a protective effect of repeated FVIII infusions (ITI) on bleeding in patients who have developed inhibitors; these data should be used to plan ITI and/or serve as a comparator for prophylaxis with nonreplacement therapy.
Introduction
Modern treatment for severe hemophilia A is based on prevention of bleeding and subsequent arthropathy by regular replacement therapy with factor VIII (FVIII) or with nonreplacement therapy.1 Unfortunately, ∼30% of young boys with severe hemophilia A (FVIII level < 0.01 IU/dL) develop allo-antibodies (inhibitors) to FVIII concentrates.2 Inhibitors render FVIII prophylaxis ineffective, and patients who develop inhibitors, especially patients with high-titer inhibitors (≥5 Bethesda units [BU]/mL) need treatment with bypassing agents in case of bleeding or procedures. Therefore, the current international and national guidelines still recommend an attempt at immune tolerance induction (ITI) therapy for patients with severe hemophilia A who developed inhibitors although effective prophylaxis can nowadays be achieved using nonreplacement (emicizumab) therapy.1,3-6
ITI is the only proven treatment for inhibitor eradication comprising repeated administration of FVIII and is successful for ∼70% of patients.7 ITI regimens vary according to both dose and frequency, from FVIII 25-50 IU/kg 3 times weekly to 100-150 IU/kg twice daily. The randomized international ITI study compared low-dose/low-frequency ITI (50 IU/kg, 3 days per week) with high-dose/high-frequency ITI (200 IU/kg daily) for children with high-titer inhibitors. It showed equal effectiveness but was stopped prematurely because of increased bleeding in patients treated with the low-dose/low-frequency regimen.8 The choice of when to start ITI and which regimen for a child with a newly diagnosed inhibitor, therefore, needs to balance the burden of frequent infusions with the need to eradicate the inhibitor and the risk of bleeding.
The international prospective multicenter PedNet (European Network for Hemophilia Management) cohort includes data from 16 countries with access to ITI and a wide variation of treatment strategies. Using this cohort, this study presents an analysis of bleeding in patients with high-titer inhibitors, from inhibitor detection to the start of ITI and during ITI. These data are essential to plan bleeding control and tolerance induction in centers without access to emicizumab prophylaxis and serve as a comparator for those with emicizumab available.
Patients and methods
Study design and patients
The prospective PedNet Registry9 was established to evaluate treatment, side effects, and outcome of hemophilia. It is a prospective cohort study that includes all patients born since 2000 with hemophilia with clotting factor activities between <0.01 and 0.25 BU/mL, from 32 hemophilia treatment centers in Europe, Israel, and Canada. It includes follow-up, at the participating centers, for all patients from diagnosis until the age of 18 years. All details on the first 50 exposure days (EDs) are collected, followed by annual evaluations of treatment, side effects, and outcome. This includes collection of results from all inhibitor tests performed. According to protocol, inhibitor tests were performed at least every 5 EDs in the first 20 EDs, followed by every 10 EDs until ED50, and at least annually thereafter. In 2023, the PedNet cohort included 2759 patients. From this cohort, all patients with severe hemophilia A (FVIII level < 0.01 IU/mL) who developed high-titer inhibitors (peak titer > 5 BU/mL) during the first 50 EDs and were born between 1 January 2000 and 31 December 2019 were eligible. Data from 31 hemophilia treatment centers (HTCs) from 16 countries participating in PedNet were included in this study. The present analysis has considered data on bleeding and treatment according to 2 time periods: “pre-ITI,” that is, from the first positive inhibitor titer until the start of ITI, and “during ITI,” defined as the period from the start of ITI until the first negative inhibitor titer result, until discontinuation of ITI, or the last follow-up since the start of ITI, if the inhibitor was still present. Data on treatment and bleeding were updated until 1 January 2021.
Ethical aspects
All participating HTCs obtained the approval of their local ethical committees according to local regulations. A signed informed consent was obtained from all parents or caregivers before the inclusion in the PedNet Registry. Data processing, storage, and monitoring were performed as previously reported.9
Data collection
Data on patient characteristics, bleeding, and treatment were collected from the medical records by using a standardized electronic data capture system, whereas laboratory results were retrieved from original local laboratory reports.
Patient characteristics included age and EDs to FVIII at the time of first positive inhibitor titer and age at first negative inhibitor titer. Clinical data included the total number of bleeds and the number of joint bleeds and life-threatening bleeds, without specification of etiology (traumatic, spontaneous, etc). Individual treatment data included dose and frequency of ITI and bypassing agents (BPAs) during the total follow-up period. Laboratory data included inhibitor peak titer ever reached, pre-ITI inhibitor peak titer, and inhibitor peak titer during ITI. Inhibitor tests were performed at the local laboratories according to the Nijmegen modification.10
Definitions
Definitions were based on consensus among PedNet members at the time of the first longitudinal analysis of patients with low-titer inhibitors.11
Clinically relevant inhibitors: 2 positive inhibitor titers detected on subsequent plasma samples in association with a decreased FVIII recovery or 1 positive inhibitor result with abnormal response to FVIII and/or need for treatment with BPAs.
Peak inhibitor titer: highest inhibitor titer measured during follow-up. For summary data, the peak inhibitor titer was defined as the highest titer during follow-up. For analyses of bleeding according to treatment, the peak inhibitor titer reflects the highest titer measured during that specific treatment period.
High-titer inhibitors: peak inhibitor titer ≥ 5 BU/mL at least once during follow-up.
ITI: any regular FVIII infusion schedule given at least 3 times per week and at a dose ≥ 45 IU/kg per infusion for a minimum of 4 weeks.
ITI frequency: nondaily vs daily.
ITI dose: low dose (≥45-<95 IU/kg per day) vs high dose (≥95 IU/kg per day). Of note, this definition was adapted from the original definition (ie, high dose ≥ 100 IU/kg per day)11 to better reflect the intention of the treater and small variations in weight; it was considered that a dose of 98 IU/kg represents the intention to administer 100 IU/kg.
Prophylaxis with bypassing agents: infusions ≥ 2 times per week (minimum duration, 3 months) with either recombinant activated factor VII (rFVIIa) or activated prothrombin complex concentrate (aPCC).
Joint bleed: any complaint located in a joint requiring treatment with FVIII and/or BPAs.
Life-threatening bleed: according to the PedNet data collection protocol, any potentially life- or limb-threatening bleed according to the treating physician, including intracranial bleeds.
Total bleeding: all registered bleeds requiring treatment with FVIII and/or BPAs.
Statistical analysis
Because the aim of the study was to compare bleeding according to inhibitor titer and treatment, follow-up periods were split and analyzed according to the treatment type received, that is, receipt of ITI, yes/no; prophylaxis with BPA, yes/no; and dose and frequency of ITI. To account for differences in follow-up, mean annual bleeding frequencies and 95% confidence intervals (CIs) were calculated using negative binomial regression modeling.12 Consequently, the same patient could contribute data to various treatment strategies, for example, 8 months before ITI and 24 months during ITI. Only treatment periods with data available on bleeding were considered.
To promote interpretation and future analyses, peak inhibitor titers were analyzed according to categories. Categories were based on literature regarding ITI prognosis and current protocols on ITI3,4,13 Bleeding was compared according to the highest inhibitor titer during the period considered in 3 groups (5-39 BU, 40-199 BU, and ≥200 BU) and according to various ITI regimens (low dose nondaily; low dose daily; high dose nondaily; and high dose daily).
Patients receiving prophylaxis with BPAs were compared with those who did not receive BPA prophylaxis but were on the same ITI regimen.
The independent associations of ITI dose, ITI frequency, BPA prophylaxis, and inhibitor titer with annual bleeding rate (ABR) and annual joint bleeding rate (AJBR) during ITI were studied using multivariable negative binomial regression.
Continuous variables, expressed as median values and interquartile ranges (IQRs; 25th percentile to 75th percentile), were compared by the Student t test or the Mann-Whitney U test according to their distribution. Comparisons of >2 groups were performed using independent sample Kruskall Wallis tests. Categorical variables, expressed as frequencies and percentage values, were compared by χ2 tests.
All reported P values are 2-sided, and values <.05 were considered significant. All analyses were performed using Statistical Package for the Social Sciences Statistics software (release 26.0, International Business Machines Corporation, Armonk, NY).
Results
Patients
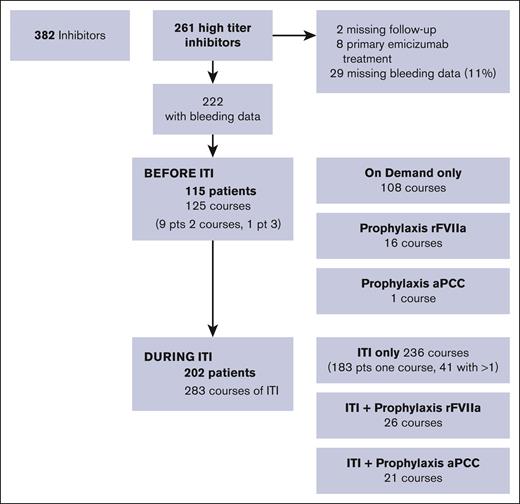
Data on patients with severe hemophilia A, born from 2000 to 2019, from the international PedNet Registry were analyzed with a cut-off date of 1 January 2021. An overview of available data is shown in Figure 1. The registry included 382 patients with severe hemophilia A with clinically relevant inhibitors, including 261 with high-responding inhibitors. After exclusion of 2 patients with missing follow-up data, 8 who immediately started emicizumab treatment and 29 (11%) with missing data on bleeding, 222 patients were available for analysis.
Flow diagram of available data on patients with high-titer inhibitors, born from 2000 to 2019.
Flow diagram of available data on patients with high-titer inhibitors, born from 2000 to 2019.
The eventual 222 patients with high-responding inhibitors were followed up for a total of 408 patient years, with a median of 1.72 years (IQR, 0.8-2.9) per patient, starting at age 1.15 years (IQR, 0.78-1.51). Patients were categorized according to their peak inhibitor titer, with 40% at 6 to 39 BU, 25% at 40 to 199 BU, and 35% at very high titers of ≥200 BU. Overall, 2025 bleeds were registered, including 951 joint bleeds, 3 intracranial bleeds, and 3 other life- or limb-threatening bleeds.
Bleeding was analyzed according to different treatment courses, that is, a period with a certain treatment (Figure 1). For example, 1 patient could contribute both a period of on-demand treatment before ITI and 2 courses of ITI with different regimens. In total, 115 patients had data on bleeding during 125 treatment courses before ITI, including 108 without prophylaxis with BPAs, 16 with rFVIIa prophylaxis, and 1 with aPCC prophylaxis. During ITI, data were available for 283 courses of ITI for 202 patients, including 236 without BPA prophylaxis, 26 combined with rFVIIa prophylaxis, and 21 combined with aPCC prophylaxis.
Overall bleeding rates before ITI and during ITI showed that ITI resulted in lower bleeding rates: the ABR was 6.12 (95% CI, 4.97-7.54) before ITI vs 4.43 (95% CI, 3.85-5.09) during ITI (P = .006), whereas joint bleeding rates were reduced from 2.76 (95% CI, 2.21-3.46) before ITI to 1.73 (95% CI, 1.49-2.01) during ITI (P = .003).
Bleeding before ITI
Treatment characteristics and bleeding according to prophylaxis with BPAs before ITI are shown in Table 1. Overall follow-up before starting ITI was relatively short, at a median of 0.71 years. Before starting ITI, prophylaxis with BPAs was administered 2 to 3 times weekly, most likely to patients with frequent bleeding, resulting in similar bleeding rates among those with and without BPA prophylaxis.
Bleeding according to the peak inhibitor titer is shown in Table 2. ABR, especially before ITI, was independent of peak inhibitor titer. AJBR showed a nonsignificant trend of increased bleeding for those with the highest inhibitor titers.
ITI regimens
Treatment characteristics and bleeding of 202 patients receiving 283 ITI treatment courses are shown in Table 3. ITI was started at a median age of 2.3 years, and the treatment courses had a median duration of 0.85 years. The majority of patients received high-dose daily ITI (63.9%), followed by low-dose nondaily in 17.3%, high-dose nondaily in 17.0%, and low-dose daily in 1.8%. BPA prophylaxis was prescribed for 25.2% of patients, for a median duration of ∼1 year, almost equally divided between rFVIIa and aPCC. Prophylaxis with rFVIIa was mainly administered 3 times, weekly, whereas prophylaxis with aPCC was generally administered twice daily. Details of the ITI regimens are shown in Table 4. The median duration of these courses was similar, at only <1 year, the median inhibitor titers appeared lower in the low-dose regimens, and the median weekly dose of FVIII varied widely from 176 to 1400 IU/kg per week. The independent associations of dose and frequency of FVIII treatment with bleeding rates were studied using multivariable regression analyses.
Bleeding according to inhibitor titer during ITI
During ITI, bleeding rates showed a clear association with peak inhibitor titer. Both overall bleeding and joint bleeding were significantly increased in patients with higher peak inhibitor titers: mean ABR increased from 2.32 (95% CI, 1.78-3.02) in patients with a peak inhibitor titer < 40 BU to 4.17 (95% CI, 3.48-6.39) in those with titers ranging from 40 to 199 BU and to 5.76 (95% CI, 4.51-7.36) in those with titers ≥ 200 BU. Similarly, mean AJBR increased from 0.66 (95% CI, 0.47-0.92) to 1.52 (95% CI, 1.09-2.13) and to 2.86 (95% CI, 2.20-3.71), respectively.
Bleeding according to ITI regimen
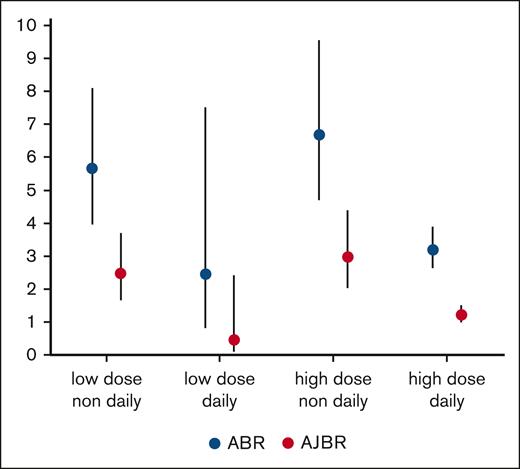
An overview of annualized joint bleeding according to ITI regimen is shown in Figure 2A-B. This univariable comparison showed that both overall bleeding and joint bleeding were similar across different daily doses of ITI (P = .88 for overall bleeding and P = .26 for joint bleeding), whereas the frequency of ITI infusions showed a statistically and clinically significant association with bleeding rates. The mean ABR was 6.09 per year (95% CI, 4.73-7.83) during nondaily ITI vs 3.33 per year (95% CI, 2.68-4.15) during daily ITI (P < .001). The mean AJBR was 2.69 per year (95% CI, 2.04-3.54) during nondaily ITI and 1.40 per year (95% CI, 1.10-1.78) during daily ITI (P < .01). ABR was similar for patients receiving once-daily and twice-daily ITI infusions: 3.33 per year (95% CI, 2.68-4.15) vs 2.63 per year (95% CI, 1.76-3.98) for total bleeds (P = .323), whereas AJBR was significantly reduced from 1.40 per year (95% CI, 1.10-1.78) during once daily ITI to 0.59 (95% CI, 0.35-0.98) during twice-daily ITI (P = .003). As expected, differences in infusion frequency were also associated with large differences in ITI dose: from median 91 IU/kg per day in the nondaily group to 200 IU/kg per day in the daily group and 213 IU/kg per day in the twice-daily group (P < .001). To study the independent associations of treatment parameters and inhibitor titer with ABR and AJBR corrected for differences in the follow-up, we performed multivariable regression modeling as shown in Table 5. All parameters included are shown in Table 5; age was not included because its variation was very limited.
Both analyses of ABR and AJBR showed 2.7- to 3.0-times increased bleeding with peak inhibitor titers ≥40 BU and 0.44- to 0.45-times reduced bleeding with daily ITI infusions (vs nondaily) but no reduction in bleeding with daily ITI doses ≥95 IU/kg per day. Findings for BPA prophylaxis showed no reduction in ABR or AJBR for rFVIIa prophylaxis (generally administered 3 times per week) during ITI but showed a trend toward ABR reduction (adjusted hazard ratio [aHR], 0.68) and a significant AJBR reduction (aHR, 0.37; 95% CI, 0.18-0.77) for aPCC prophylaxis, which was administered twice daily for majority of the patients. For interpretation, it must be emphasized that the regression analysis yields the independent association of each parameter with bleeding; for example, the association of bleeding with inhibitor titer presented is independent of different treatments received.
Sensitivity analyses without BPA prophylaxis and another analysis considering only the first course of ITI yielded similar aHRs for inhibitor titer and ITI dose and frequency.
Intracranial and life- or limb-threatening bleeds
In total, 3 patients suffered intracranial bleeding: 1 before ITI (inhibitor titer >200 BU, on rFVIIa prophylaxis) and 2 during ITI at titers of 6 to 40 BU (1 on high-dose daily ITI, the other on high-dose nondaily ITI, and neither on BPA prophylaxis); information on head trauma was lacking, and none of the intracranial bleeds were fatal. Four life- or limb-threatening bleeds occurred during ITI: a throat bleed (40-199 BU on high-dose daily ITI), a muscle bleed leading to compartment syndrome, a pleural hemorrhage (both at ≥200 BU on high-dose nondaily ITI), and a retroperitoneal hematoma (≥200 BU on high-dose daily ITI), all without BPA prophylaxis.
Discussion
Summary
With 222 patients, this study presents, to our knowledge, the largest data set on bleeding rates before and during ITI in patients with severe hemophilia A and high-titer inhibitors in the absence of nonreplacement therapy. ITI treatment significantly reduced both mean ABR (from 6.06 to 4.43 per year) and AJBR (from 2.62 to 1.73 per year).
Before ITI, bleeding rates in these patients with inhibitors were independent of inhibitor titer.
During ITI, bleeding rates were higher with high inhibitor titer and nondaily ITI but were not associated with ITI dose.
These findings provide a comparator for bleeding rates in young patients with inhibitors, and suggest a protective effect of daily short peaks of FVIII despite the presence of an inhibitor.
Internal and external validity
This study is part of a large prospective observational cohort study. All patients with a clinically relevant inhibitor (ie, 2 positive titers and reduced recovery of FVIII) were followed up from the first positive inhibitor titer until the first negative titer. Although it is well known that the first negative titer does not coincide with the normalization of FVIII half-life, we considered this the best end point for the evaluation of bleeding during the presence of an inhibitor. Bleeding data were extracted from medical files, collected at infusion level, but were well documented because most of these very young patients (median age at inhibitor diagnosis, 1.15 years) visited the treatment center at the time of bleeding and many were still in the first 50 EDs to FVIII. Regarding the bleeding before ITI, it is logical that bleeding rates were not associated with inhibitor titer because all of these patients had the same FVIII activity level of <0.01 IU/mL. The AJBR data may suggest a trend toward more joint bleeding before ITI in patients with high-titer inhibitors, but this is likely a “chance-finding” because inhibitor activity does not change coagulation potential in the absence of replacement therapy, and bleeds at this young age are most likely traumatic.
ABR and AJBR were modeled using negative binomial regression that accounts for variation in follow-up as well as the skewed distribution of the data.12 This technique is used in most recent studies14 and allows for comparison with the present data. It is clear that high-dose ITI was preferentially prescribed to those with the highest inhibitor titers, and, especially daily ITI was almost exclusively administered at high doses. For nondaily ITI, however, the proportions of patients receiving low and high doses were equal. In these cases, multivariable regression analysis, adjusting for inhibitor titer, ITI dose, and ITI frequency simultaneously, is the only way to unravel the independent contributions of the various determinants.
Because this cohort of patients with high-titer inhibitors is part of the PedNet cohort of patients with severe hemophilia from 31 HTCs, many treatment strategies are represented, which results in high external validity. All patients included had access to ITI and treatment with BPAs.
Comparison with others
Data on bleeding frequency in very young children with inhibitors are scarce.
Bleeding in the absence of ITI was mostly reported by studies on BPA prophylaxis15-17 or as a comparison before starting emicizumab prophylaxis in selected patients with frequent bleeding.18 The PROPACT study16 reported on a retrospective analysis of 86 patients from 42 sites in 14 countries who started rFVIIa prophylaxis at a median age of 6 years. In the absence of ITI and BPA prophylaxis, bleeding rates reported were much higher than in this study (15.7 per year [95% CI, 14.2-17.4] vs 5.9 per year [95% CI, 4.8-9.4]) but similar during daily rFVIIa prophylaxis at 8.2 per year (95% CI, 7.6-8.9) in the PROPACT study vs 6.7 per year (95% CI, 3.9-11.6) on rFVIIa prophylaxis in this study.
The best comparison of bleeding during ITI is with the landmark international ITI study,8 which randomized patients with severe hemophilia A with an inhibitor titer of 5 to 200 BU at a median age of 1.2 years, into low-dose nondaily (n = 58) or high-dose daily ITI (n = 57). Cox modeling of bleeding until the first negative titer showed a significantly higher mean ABR of 7.5 per year in the low-dose arm vs 3.4 per year in the high-dose arm (P < .001). These findings are similar to our findings of a mean ABR of 5.7 per year (95% CI, 4.0-8.1) on low-dose nondaily ITI and a mean ABR of 3.2 per year (95% CI, 2.6-3.9) in 158 patients receiving high-dose daily ITI.
A similar mean ABR of 5.4 per year was recently reported on an interim analysis of the first 100 patients of the OBSITI study on ITI with plasma-derived FVIII in patients with inhibitors with a poor prognosis.19 In the aforementioned study, the majority of patients (86%) had a high-responding inhibitor and received high-dose daily ITI, but CIs and bleeding according to different treatment strategies were not provided.
Interpretation and clinical implications
These data show that bleeding before ITI is independent of inhibitor titer, but during ITI it increases with inhibitor titer and decreases with daily FVIII infusions. This suggests that frequent peaks of FVIII activity have some protective effect on bleeding in these patients despite the extremely short half-life of FVIII in the presence of a high-responding inhibitor. This is supported by in vitro studies reporting that addition of FVIII to rFVIIa or aPCC to plasma from patients with a high-responding inhibitor improves global hemostatic parameters.20,21 The observation that inhibitor titer is an independent determinant of joint bleeding during ITI suggests a dose response: the titer determines the duration of FVIII in circulation, and, apparently, half-life is a stronger determinant than dose. Consequently, bleeding control was best in daily ITI regimens.
How can these findings be applied in clinical management?
Directly after diagnosis of an inhibitor, effective prophylaxis can be established with emicizumab, which is more convenient and appears more effective than BPA prophylaxis, although a head-to-head comparison between the 2 is currently lacking.
For the choice of ITI regimen, it is important to acknowledge that daily ITI is associated with reduced bleeding. Combined with emicizumab, however, daily ITI is unlikely to provide added value in bleeding control.8
Independent of the issue of inhibitor eradication, the present data may serve as a comparator for the evaluation of bleeding and prophylactic treatment in boys with severe hemophilia A with an inhibitor.
The comparison between prophylaxis with rFVIIa and aPCC before ITI is inconclusive because it is limited by low numbers and, during ITI, by the difference in administration frequency (3 times per week vs twice daily), which was not included in the multivariable regression analysis. However, it is likely that the high frequency of aPCC administration has provided a considerable contribution to its effectiveness, and this must be considered when interpreting these findings.
Future research
The choice of an ITI regimen should balance its success rate and its clinical and economic burden. Using emicizumab in lieu of ITI has been well described for pediatric patients,14 and emicizumab prophylaxis may even be considered a cost-saving treatment for patients with hemophilia A with inhibitor during ITI.22,23 To facilitate the choice of an ITI regimen in the absence of emicizumab prophylaxis, future studies should combine data on predictors of ITI success, the effectiveness of ITI, bleeding, preferred regimens, and treatment costs.
A more definitive answer on the question whether bleeding is increased because of inhibitory activity in the absence of regular FVIII treatment may be provided by performing a nested case-control study within the PedNet cohort matched for known predictors of bleeding.
Conclusions
For 222 patients with severe hemophilia A and high-titer inhibitors followed up for a median of 1.72 years, mean AJBR was significantly reduced from 2.62 per year (95% CI, 2.11-3.24) before ITI to 1.73 per year (95% CI, 1.49-2.01) during any ITI.
Inhibitor titers ≥40 BU were associated with increased bleeding during ITI only. During ITI, daily FVIII infusions were associated with reduced bleeding, but ITI dose showed no association with bleeding.
These findings suggest a protective effect of daily FVIII infusions despite the presence of an inhibitor and can be used as a comparator for bleeding on nonreplacement therapy.
Acknowledgments
The authors are indebted to all PedNet members (supplemental Appendix), participating centers, and data managers for their continuing support over a 20-year period, collecting and sharing detailed clinical data. The authors are grateful to the PedNet study staff for their support in data management, and especially to Elsbeth de Boer-Verdonk for data preparation.
The PedNet Registry is owned by the PedNet Haemophilia Research Foundation, a not-for-profit foundation that has received unrestricted research grants from Bayer AG, Takeda, Novo Nordisk, CSL Behring, Pfizer Inc, Biotest AG, Swedish Orphan Biovitrum AB, LFB Biotechnologies, Sanofi, and Hoffmann-La Roche. Companies supporting the PedNet Registry receive annual aggregated data on safety and efficacy of their concentrates. None of the authors received any funding for work done in relation to this manuscript.
Authorship
Contribution: K.F., C.K., and K.K. designed the study and wrote the first draft of the manuscript; K.F. performed the statistical analyses; and all authors performed research, participated in data interpretation and writing of the manuscript, and approved of the final version.
Conflict-of-interest disclosure: The institution of K.F. has received speaker’s fees from CSL Behring and Novo Nordisk; consultancy fees from Biogen, CSL Behring, Freeline, Novo Nordisk, Roche, and Sobi; and research support from Bayer, Pfizer, and Novo Nordisk. G.K. has received research support from Alnylam, Bayer, Opko, Pfizer, Roche, and Takeda, and consultancy fees from ASC Therapeutics, Bayer, BioMarin, CSL Behring, Novo Nordisk, Pfizer, Roche, Takeda, and UniQure. K.K. has received research funding from Bayer, CSL Behring, Swedish Orphan Biovitrum, and Shire/Takeda, and consultancy, honoraria, scientific advisory board, and/or travel expenses from Bayer, Biotest, Chugai/Roche, CSL Behring, Swedish Orphan Biovitrum and Shire/Takeda. M.C. has received research support from Bayer, Bioverativ/Sanofi, CSL Behring, Novo Nordisk, Octapharma, Pfizer, and Shire/Takeda, and speaker’s/consultancy fees from Bayer, Bioverativ/Sanofi, Biotest, CSL Behring, Grifols, LFB, Novo Nordisk, Octapharma, Pfizer, Roche, and Shire/Takeda. J.O. has received research funding from Bayer, Biotest, CSL Behring, Octapharma, Pfizer, Swedish Orphan Biovitrum, and Takeda, and consultancy, speakers bureau, honoraria, scientific advisory board, and travel expenses from Bayer, Biogen Idec, BioMarin, Biotest, Chugai, CSL Behring, Freeline, Grifols, LFB, Novo Nordisk, Octapharma, Pfizer, Roche, Sanofi, Spark Therapeutics, Swedish Orphan Biovitrum, and Takeda. A.R.C.H. has received speaker fees from Novo Nordisk and Stago. J.B. has received consultancy and/or speaker fees from Bayer, LFB, Novo Nordisk, Pfizer, Roche, Shire/Takeda, and Sobi. C.K. has received personal fees from BayerVital GmbH, CSL Behring, Novo Nordisk, Roche/Chugai, Sanofi/Sobi, and Takeda, and grants (to institution) from Bayer VitalGmbH, Biotest, CSL Behring, Intersero, Novo Nordisk, Pfizer, Sanofi/Sobi, and Takeda. The remaining authors declare no competing financial interests.
A complete list of the members of the PedNet Study Group appears in the supplemental Appendix.
Correspondence: Kathelijn Fischer, Center for Benign Hematology, Thrombosis and Hemostasis, Van Creveldkliniek, University Medical Center Utrecht, Utrecht University, Room C01.428, PO Box 85500, 3508 GA Utrecht, The Netherlands; email: k.fischer@umcutrecht.nl.
References
Author notes
Restrictions apply to the availability of the data, which were used under license for this study. Data that support the findings of this study are available with permission from the PedNet Haemophilia Research Foundation at www.pednet.eu.
The full-text version of this article contains a data supplement.