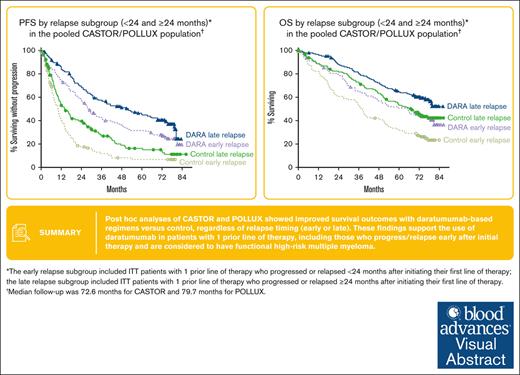
Post hoc analyses of CASTOR/POLLUX showed PFS and depth of response benefit with daratumumab, regardless of relapse timing (early or late).
These results support daratumumab use for patients with MM who relapse early after initial therapy and have functional high-risk disease.
Visual Abstract
High-risk multiple myeloma (MM) is often defined based on cytogenetic abnormalities, but patients who relapse early after initial therapy are considered a functional high-risk group. In the phase 3 CASTOR and POLLUX studies, daratumumab plus bortezomib/dexamethasone (D-Vd) or lenalidomide/dexamethasone (D-Rd) improved progression-free survival (PFS) and overall survival (OS), regardless of cytogenetic risk, and achieved higher rates of complete response or better (≥CR) and minimal residual disease (MRD) negativity vs that with Vd/Rd alone in relapsed/refractory MM. Post hoc analyses of CASTOR and POLLUX evaluated patient subgroups with 1 prior line of therapy based on timing of progression/relapse (early or late) after initiation of first line of therapy. PFS consistently favored the daratumumab-containing regimens across subgroups using both a 24- and 18-month early-relapse cutoff. In the CASTOR/POLLUX pooled data set, daratumumab reduced the risk of disease progression or death by 65% (hazard ratio [HR], 0.35; 95% confidence interval [CI], 0.26-0.48; P < .0001) in the early-relapse (<24 months) subgroup and by 65% (HR, 0.35; 95% CI, 0.26-0.47; P < .0001) in the late-relapse (≥24 months) subgroup. OS also favored the daratumumab-containing regimens in both the early-relapse (HR, 0.62; 95% CI, 0.45-0.86; P = .0036) and late-relapse (HR, 0.67; 95% CI, 0.48-0.93; P = .0183) subgroups in the pooled population using a 24-month cutoff. Rates of ≥CR and MRD negativity (10−5) were higher with daratumumab vs control, regardless of progression/relapse timing. Although daratumumab is unable to fully overcome the adverse prognosis of early relapse, our results support the use of daratumumab for patients with 1 prior line of therapy, including for those who progress/relapse early after initial therapy and are considered to have functional high-risk MM. These trials were registered at www.clinicaltrials.gov as #NCT02136134 (CASTOR) and #NCT02076009 (POLLUX).
Introduction
High-risk multiple myeloma (MM) is often defined based on the presence of cytogenetic abnormalities that have been associated with worse prognosis, namely t(4;14), t(14;16), t(14;20), gain(1q21)/amp(1q21), and/or del17p.1,2 However, patients with MM who relapse early after initial therapy, even in the absence of ≥1 of these features, are considered a functional high-risk group that is also associated with poor prognosis.1,3-6 A genomic analysis of patients with newly diagnosed MM (NDMM) in the Multiple Myeloma Research Foundation CoMMpass data set divided patients into 3 groups: a genomic high-risk group (defined as patients with t[4;14] or t[14;16] or complete loss of functional TP53 or 1q21 gain and International Staging System [ISS] stage III), a functional high-risk group (defined as patients with no markers of the genomic high-risk group but who were refractory to induction therapy or had early relapse within 12 months), and a standard-risk group (defined as patients who did not fulfill any of the criteria for genomic or functional high risk).4 Interestingly, patients in the functional high-risk group who had no clinically applied high-risk genetic factors had the poorest median overall survival (OS; 27.6 months) compared with those in the standard-risk group (median, not reached [NR]) or the genomic high-risk group (44.7 months).4 Patients with functional high-risk MM represent an ongoing unmet therapeutic need.
Daratumumab is a human immunoglobulin Gκ monoclonal antibody targeting CD38 with a direct on-tumor7-10 and immunomodulatory11-13 mechanism of action, demonstrating greater cytotoxicity toward MM cells ex vivo compared with analogs of other CD38 antibodies.14 Daratumumab induces higher levels of complement-dependent cytotoxicity and similar levels of antibody-dependent cell-mediated cytotoxicity and antibody-dependent cellular phagocytosis and, in the presence of Fc receptor crosslinking, which occurs naturally in vivo, daratumumab elicits similar levels of cell death compared with analogs of other CD38 antibodies.14 Results from phase 3 studies demonstrated that the addition of daratumumab to standard-of-care regimens improved progression-free survival (PFS) and OS and achieved deep and durable responses for patients with MM.15-25 Daratumumab is approved as monotherapy for relapsed or refractory MM (RRMM) and in combination with standard-of-care regimens for patients with RRMM or NDMM.26,27
In the phase 3 CASTOR and POLLUX studies, daratumumab in combination with bortezomib and dexamethasone (D-Vd) or lenalidomide and dexamethasone (D-Rd), respectively, improved PFS, regardless of cytogenetic risk status, and achieved higher rates of complete response or better (≥CR) and minimal residual disease (MRD) negativity vs Vd or Rd alone for patients with RRMM.21,23 Recently reported survival data from CASTOR and POLLUX demonstrated, to our knowledge, for the first time, an OS benefit with daratumumab-containing regimens in RRMM, including for patients with high-risk cytogenetic abnormalities.22,24
Here, we present post hoc analyses of CASTOR and POLLUX conducted to evaluate D-Vd vs Vd and D-Rd vs Rd in patient subgroups with 1 prior line of therapy based on timing of progression or relapse (early or late) after initiation of the first line of therapy to determine the efficacy of daratumumab-containing regimens in functional high-risk MM.
Methods
Trial design and oversight
CASTOR (NCT02136134) and POLLUX (NCT02076009) are multicenter, randomized, open-label, phase 3 studies in patients with RRMM. The study designs have been published previously.28,29 Briefly, eligible patients in both studies had received ≥1 prior line of therapy, achieved at least a partial response to ≥1 previous therapy, and documented progressive disease per International Myeloma Working Group criteria30,31 on or after their last regimen. Patients refractory to or intolerant of bortezomib or refractory to another proteasome inhibitor were excluded from CASTOR.28 Patients refractory to or intolerant of lenalidomide were excluded from POLLUX.29 Trial protocols were approved by independent ethics committees or institutional review boards at each site. Patients provided written informed consent, and both trials were conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines.
Randomization and study treatment
Patients were randomly assigned 1:1 to D-Vd or Vd in CASTOR and to D-Rd or Rd in POLLUX.28,29 In CASTOR, patients were stratified based on ISS disease stage (I vs II vs III), number of prior lines of therapy (1 vs 2 or 3 vs >3), and previous bortezomib treatment (yes vs no). All patients received up to 8 cycles (21 days per cycle) of bortezomib (1.3 mg/m2 subcutaneously on days 1, 4, 8, and 11) and dexamethasone (20 mg orally or IV on days 1, 2, 4, 5, 8, 9, 11, and 12). Patients in the D-Vd group received daratumumab (16 mg/kg IV) weekly in cycles 1 to 3, every 3 weeks in cycles 4 to 8, and every 4 weeks thereafter until disease progression or unacceptable toxicity.
In POLLUX, patients were stratified based on ISS disease stage (I vs II vs III), number of lines of previous therapy (1 vs 2 or 3 vs >3), and previous lenalidomide treatment (yes vs no). All patients received 28-day cycles of lenalidomide (25 mg orally on days 1-21 of each cycle; 10 mg if creatinine clearance was 30-60 mL/min) and dexamethasone (40 mg weekly) until disease progression or unacceptable toxicity. For patients in the D-Rd group, daratumumab (16 mg/kg IV) was administered weekly in cycles 1 and 2, every 2 weeks during cycles 3 through 6, and every 4 weeks thereafter.
End points and assessments
In both studies, PFS was the primary end point and was defined as the duration from the date of randomization to the date of disease progression or death, whichever occurred first. Secondary end points included overall response rate (ORR), very good partial response or better (≥VGPR) and MRD-negativity rates, and OS. Response and disease progression were assessed by a validated computer algorithm, based on International Myeloma Working Group criteria.30,31 MRD was assessed using bone marrow aspirate samples and evaluated via a next-generation sequencing approach using the clonoSEQ assay (version 2.0; Adaptive Biotechnologies, Seattle, WA). In this analysis, the early-relapse subgroup included patients with 1 prior line of therapy who progressed or relapsed <24 months after initiating their first line of therapy; the late-relapse subgroup included patients with 1 prior line of therapy who progressed or relapsed ≥24 months after initiating their first line of therapy. In a second analysis, the early-relapse subgroup was defined as patients with 1 prior line of therapy who progressed or relapsed <18 months after initiating their first line of therapy; the late-relapse subgroup included patients with 1 prior line of therapy who progressed or relapsed ≥18 months after initiating their first line of therapy.
Statistical analyses
Statistical methods have been published previously.21,23,28,29 Time-to-event end points were estimated using the Kaplan-Meier method. Hazard ratios (HRs), 95% confidence intervals (CIs), and P values were estimated using a Cox regression model, with treatment as the sole explanatory variable (stratified according to study ID for pooled analyses). ORRs and ≥VGPR and ≥CR rates were compared using the Cochran–Mantel-Haenszel test (stratified according to the study ID for pooled analyses). MRD-negativity rates were compared using Mantel-Haenszel estimate of the common odds ratio (stratified according to the study ID for pooled analyses), with the P value from a Fisher exact test. Data are presented for both the 24- and 18-month cutoffs defining early vs late relapse, respectively. Results have been pooled for CASTOR and POLLUX combined and are also presented for each study individually.
Results
Patients
A total of 240 and 290 patients from the pooled CASTOR/POLLUX data set were included in the early-relapse (<24 months) and late-relapse (≥24 months) subgroups, respectively. Patient baseline characteristics by relapse subgroup are summarized in Table 1. Baseline demographics and clinical characteristics for the pooled CASTOR/POLLUX data set using the 18-month early/late-relapse cutoff are shown in supplemental Table 1, and patient data for each individual study are shown in supplemental Table 2 (CASTOR) and supplemental Table 3 (POLLUX). Median time from initiation of the prior line of therapy to progression/relapse was similar between treatment arms (D-Vd vs Vd; D-Rd vs Rd) within the early- and late-relapse groups for the 24-month and 18-month cutoffs (supplemental Table 4).
Baseline demographic and clinical characteristics according to the relapse subgroup
| . | Early relapse (<24 mos) . | Late relapse (≥24 mos) . | ||
|---|---|---|---|---|
| DARA (n = 125) . | Control (n = 115) . | DARA (n = 146) . | Control (n = 144) . | |
| Age, y | ||||
| Median (range) | 65 (30-89) | 65 (40-85) | 64 (34-84) | 64 (42-85) |
| ≥75 y, n (%) | 17 (13.6) | 17 (14.8) | 8 (5.5) | 16 (11.1) |
| ISS staging, n (%)∗ | ||||
| I | 62 (49.6) | 51 (44.3) | 73 (50.0) | 81 (56.3) |
| II | 45 (36.0) | 43 (37.4) | 45 (30.8) | 42 (29.2) |
| III | 18 (14.4) | 21 (18.3) | 28 (19.2) | 21 (14.6) |
| Cytogenetic risk, n (%)† | (n = 93) | (n = 93) | (n = 116) | (n = 96) |
| Standard | 72 (77.4) | 72 (77.4) | 94 (81.0) | 85 (88.5) |
| High | 21 (22.6) | 21 (22.6) | 22 (19.0) | 11 (11.5) |
| . | Early relapse (<24 mos) . | Late relapse (≥24 mos) . | ||
|---|---|---|---|---|
| DARA (n = 125) . | Control (n = 115) . | DARA (n = 146) . | Control (n = 144) . | |
| Age, y | ||||
| Median (range) | 65 (30-89) | 65 (40-85) | 64 (34-84) | 64 (42-85) |
| ≥75 y, n (%) | 17 (13.6) | 17 (14.8) | 8 (5.5) | 16 (11.1) |
| ISS staging, n (%)∗ | ||||
| I | 62 (49.6) | 51 (44.3) | 73 (50.0) | 81 (56.3) |
| II | 45 (36.0) | 43 (37.4) | 45 (30.8) | 42 (29.2) |
| III | 18 (14.4) | 21 (18.3) | 28 (19.2) | 21 (14.6) |
| Cytogenetic risk, n (%)† | (n = 93) | (n = 93) | (n = 116) | (n = 96) |
| Standard | 72 (77.4) | 72 (77.4) | 94 (81.0) | 85 (88.5) |
| High | 21 (22.6) | 21 (22.6) | 22 (19.0) | 11 (11.5) |
Data shown of the pooled CASTOR and POLLUX data set.
The early-relapse subgroup included ITT patients with 1 prior line of therapy who progressed or relapsed <24 months after initiating their first line of therapy; the late-relapse subgroup included ITT patients with 1 prior line of therapy who progressed or relapsed ≥24 months after initiating their first line of therapy.
DARA, daratumumab; ITT, intent-to-treat.
ISS staging was based on the combination of serum β2-microglobulin and albumin.
Cytogenetic risk was assessed locally by fluorescence in situ hybridization or karyotype testing; high risk was defined as the presence of t(4;14), t(14;16), or del17p.
Efficacy
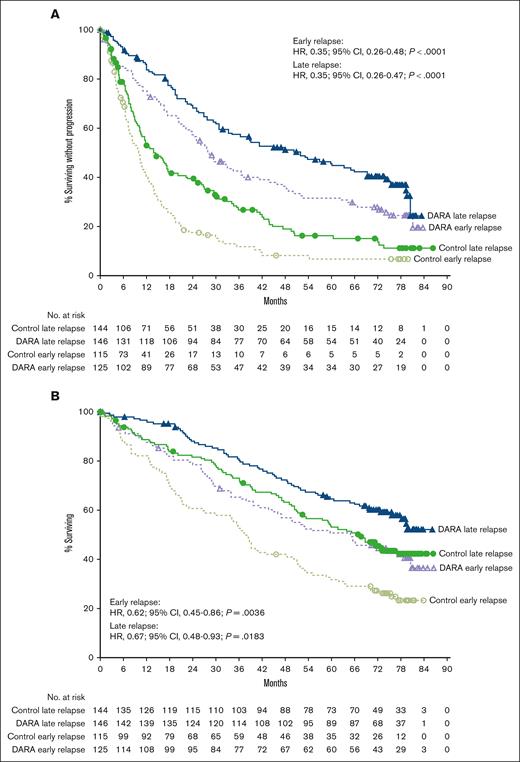
Median follow-up in the intent-to-treat population was 72.6 months (range, 0-79.8 months) for CASTOR and 79.7 months (range, 0-86.5 months) for POLLUX. In the CASTOR/POLLUX pooled population, median PFS with daratumumab vs control was 27.9 vs 10.0 months (HR, 0.35; 95% CI, 0.26-0.48; P < .0001) in the early-relapse subgroup (<24 months) and 51.8 vs 14.4 months (HR, 0.35; 95% CI, 0.26-0.47; P < .0001) in the late-relapse subgroup (≥24 months; Figure 1A). Similar PFS benefit with daratumumab vs control was observed when an 18-month cutoff was used. Median PFS with daratumumab vs control was 26.2 vs 10.3 months (HR, 0.44; 95% CI, 0.30-0.64; P < .0001) in the early-relapse subgroup (<18 months) and 43.7 vs 11.8 months (HR, 0.32; 95% CI, 0.25-0.42; P < .0001) in the late-relapse subgroup (≥18 months; supplemental Figure 1A). In the CASTOR/POLLUX pooled data set, the median OS with daratumumab vs control was 65.0 vs 38.2 months (HR, 0.62; 95% CI, 0.45-0.86; P = .0036) in the early-relapse subgroup (<24 months) and NR vs 67.3 months (HR, 0.67; 95% CI, 0.48-0.93; P = .0183) in the late-relapse subgroup (≥24 months; Figure 1B). OS benefit with daratumumab vs control was also observed in the early-relapse (median OS: 47.0 vs 38.6 months, respectively; HR, 0.77; 95% CI, 0.52-1.14; P = .1839) and late-relapse (median OS: NR vs 59.1 months, respectively; HR, 0.59; 95% CI, 0.44-0.79; P = .0003) subgroups using the 18-month early-relapse cutoff (supplemental Figure 1B).
Pooled progression free and overall survival based on the 24 month cut-off. PFS (A) and OS (B) according to the relapse subgroup (<24 and ≥24 months) in the pooled CASTOR/POLLUX population. Kaplan-Meier plots of PFS and OS in patients with 1 prior line of therapy who progressed or relapsed early or late after initiation of first line of therapy. The early-relapse subgroup included intent-to-treat (ITT) patients with 1 prior line of therapy who progressed or relapsed <24 months after initiating their first line of therapy; the late-relapse subgroup included ITT patients with 1 prior line of therapy who progressed or relapsed ≥24 months after initiating their first line of therapy. DARA, daratumumab.
Pooled progression free and overall survival based on the 24 month cut-off. PFS (A) and OS (B) according to the relapse subgroup (<24 and ≥24 months) in the pooled CASTOR/POLLUX population. Kaplan-Meier plots of PFS and OS in patients with 1 prior line of therapy who progressed or relapsed early or late after initiation of first line of therapy. The early-relapse subgroup included intent-to-treat (ITT) patients with 1 prior line of therapy who progressed or relapsed <24 months after initiating their first line of therapy; the late-relapse subgroup included ITT patients with 1 prior line of therapy who progressed or relapsed ≥24 months after initiating their first line of therapy. DARA, daratumumab.
In the pooled CASTOR/POLLUX population, ≥CR rates were higher with daratumumab vs control in the early-relapse (<24 months; 46.3% vs 13.6%; P < .0001) and late-relapse (≥24 months; 57.2% vs 29.8%; P < .0001) subgroups (Table 2). Rates of ≥VGPR were also higher with daratumumab vs control in the early-relapse (77.7% vs 39.1%; P < .0001) and late-relapse (78.6% vs 58.9%; P = .0003) subgroups. MRD-negativity rates (10−5 sensitivity) were higher with daratumumab vs control, regardless of relapse timing (early [<24 months], 22.4% vs 2.6% [P <.0001]; late [≥24 months], 31.5% vs 10.4% [P < .0001]; Table 2). Rates of ≥CR, ≥VGPR, and MRD negativity (10−5 sensitivity) were also higher with daratumumab vs control in the early-relapse and late-relapse subgroups when the 18-month cutoff was used to define early relapse (supplemental Table 5).
Response rates and MRD-negativity rates according to the relapse subgroup
| . | Early relapse (<24 mos) . | Late relapse (≥24 mos) . | ||||
|---|---|---|---|---|---|---|
| DARA . | Control . | P . | DARA . | Control . | P . | |
| Response, n (%)∗ | ||||||
| Patients evaluated, n | 121 | 110 | 145 | 141 | ||
| ORR | 110 (90.9) | 81 (73.6) | .0004† | 136 (93.8) | 114 (80.9) | .0010‡ |
| ≥CR | 56 (46.3) | 15 (13.6) | <.0001† | 83 (57.2) | 42 (29.8) | <.0001† |
| sCR | 20 (16.5) | 2 (1.8) | 44 (30.3) | 21 (14.9) | ||
| CR | 36 (29.8) | 13 (11.8) | 39 (26.9) | 21 (14.9) | ||
| ≥VGPR | 94 (77.7) | 43 (39.1) | <.0001† | 114 (78.6) | 83 (58.9) | .0003† |
| VGPR | 38 (31.4) | 28 (25.5) | 31 (21.4) | 41 (29.1) | ||
| PR | 16 (13.2) | 38 (34.5) | 22 (15.2) | 31 (22.0) | ||
| MR | 3 (2.5) | 10 (9.1) | 2 (1.4) | 7 (5.0) | ||
| SD | 4 (3.3) | 15 (13.6) | 6 (4.1) | 18 (12.8) | ||
| PD | 4 (3.3) | 3 (2.7) | 0 | 2 (1.4) | ||
| NE | 0 | 1 (0.9) | 1 (0.7) | 0 | ||
| MRD (10−5)‡ | ||||||
| Patients evaluated, n | 125 | 115 | 146 | 144 | ||
| MRD negative, n (%) | 28 (22.4) | 3 (2.6) | <.0001§ | 46 (31.5) | 15 (10.4) | <.0001§ |
| . | Early relapse (<24 mos) . | Late relapse (≥24 mos) . | ||||
|---|---|---|---|---|---|---|
| DARA . | Control . | P . | DARA . | Control . | P . | |
| Response, n (%)∗ | ||||||
| Patients evaluated, n | 121 | 110 | 145 | 141 | ||
| ORR | 110 (90.9) | 81 (73.6) | .0004† | 136 (93.8) | 114 (80.9) | .0010‡ |
| ≥CR | 56 (46.3) | 15 (13.6) | <.0001† | 83 (57.2) | 42 (29.8) | <.0001† |
| sCR | 20 (16.5) | 2 (1.8) | 44 (30.3) | 21 (14.9) | ||
| CR | 36 (29.8) | 13 (11.8) | 39 (26.9) | 21 (14.9) | ||
| ≥VGPR | 94 (77.7) | 43 (39.1) | <.0001† | 114 (78.6) | 83 (58.9) | .0003† |
| VGPR | 38 (31.4) | 28 (25.5) | 31 (21.4) | 41 (29.1) | ||
| PR | 16 (13.2) | 38 (34.5) | 22 (15.2) | 31 (22.0) | ||
| MR | 3 (2.5) | 10 (9.1) | 2 (1.4) | 7 (5.0) | ||
| SD | 4 (3.3) | 15 (13.6) | 6 (4.1) | 18 (12.8) | ||
| PD | 4 (3.3) | 3 (2.7) | 0 | 2 (1.4) | ||
| NE | 0 | 1 (0.9) | 1 (0.7) | 0 | ||
| MRD (10−5)‡ | ||||||
| Patients evaluated, n | 125 | 115 | 146 | 144 | ||
| MRD negative, n (%) | 28 (22.4) | 3 (2.6) | <.0001§ | 46 (31.5) | 15 (10.4) | <.0001§ |
Data shown of the pooled CASTOR and POLLUX data set.
The early-relapse subgroup included patients with 1 prior line of therapy who progressed or relapsed <24 months after initiating their first line of therapy; the late-relapse subgroup included patients with 1 prior line of therapy who progressed or relapsed ≥24 months after initiating their first line of therapy.
DARA, daratumumab; ITT, intent-to-treat; MR, minimal response; NE, not evaluable; PD, progressive disease; PR, partial response; sCR, stringent complete response; SD, stable disease.
Response-evaluable population with 1 prior line of therapy. The response-evaluable population was defined as patients with a confirmed diagnosis of MM and measurable disease at the baseline or screening visit who received ≥1 administration of study treatment and had ≥1 postbaseline disease assessment.
P value was calculated using the Cochran-Mantel-Haenszel χ2 test stratified based on study ID.
ITT population with 1 prior line of therapy.
P value was calculated using the Fisher exact test.
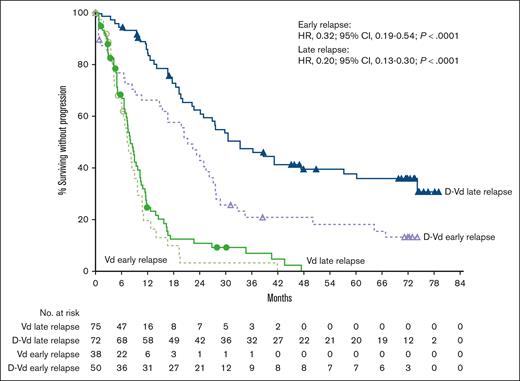
In the CASTOR study, median PFS with D-Vd vs Vd was 21.2 vs 7.3 months (HR, 0.32; 95% CI, 0.19-0.54; P < .0001) in the early-relapse subgroup (<24 months), and 33.2 vs 8.0 months (HR, 0.20; 95% CI, 0.13-0.30; P < .0001) in the late-relapse subgroup (≥24 months; Figure 2). Rates of ≥CR, ≥VGPR, and MRD negativity (10−5 sensitivity) were higher with D-Vd vs Vd in both the early-relapse (<24 months) and late-relapse (≥24 months) subgroups (Table 3). The efficacy with D-Vd vs Vd using the 18-month early-relapse cutoff was generally similar to that observed using the 24-month early-relapse cutoff (supplemental Figure 2; supplemental Table 6).
PFS by relapse subgroup (<24 and ≥24 months) in CASTOR. Kaplan-Meier plot of PFS in patients with 1 prior line of therapy who progressed or relapsed early or late after initiation of first line of therapy. The early-relapse subgroup included ITT patients with 1 prior line of therapy who progressed or relapsed <24 months after initiating their first line of therapy; the late-relapse subgroup included ITT patients with 1 prior line of therapy who progressed or relapsed ≥24 months after initiating their first line of therapy.
PFS by relapse subgroup (<24 and ≥24 months) in CASTOR. Kaplan-Meier plot of PFS in patients with 1 prior line of therapy who progressed or relapsed early or late after initiation of first line of therapy. The early-relapse subgroup included ITT patients with 1 prior line of therapy who progressed or relapsed <24 months after initiating their first line of therapy; the late-relapse subgroup included ITT patients with 1 prior line of therapy who progressed or relapsed ≥24 months after initiating their first line of therapy.
Response rates and MRD-negativity rates according to the relapse subgroup in CASTOR and POLLUX
| . | CASTOR . | |||||
|---|---|---|---|---|---|---|
| Early relapse (<24 mos) . | Late relapse (≥24 mos) . | |||||
| D-Vd . | Vd . | P . | D-Vd . | Vd . | P . | |
| Response, n (%)∗ | ||||||
| Patients evaluated, n | 47 | 36 | 72 | 73 | ||
| ORR | 39 (83.0) | 26 (72.2) | .2415† | 70 (97.2) | 55 (75.3) | .0001† |
| ≥CR | 13 (27.7) | 4 (11.1) | .0657† | 39 (54.2) | 12 (16.4) | <.0001† |
| sCR | 2 (4.3) | 0 | 16 (22.2) | 5 (6.8) | ||
| CR | 11 (23.4) | 4 (11.1) | 23 (31.9) | 7 (9.6) | ||
| ≥VGPR | 31 (66.0) | 12 (33.3) | .0034† | 60 (83.3) | 34 (46.6) | <.0001† |
| VGPR | 18 (38.3) | 8 (22.2) | 21 (29.2) | 22 (30.1) | ||
| PR | 8 (17.0) | 14 (38.9) | 10 (13.9) | 21 (28.8) | ||
| MR | 2 (4.3) | 2 (5.6) | 1 (1.4) | 6 (8.2) | ||
| SD | 2 (4.3) | 7 (19.4) | 1 (1.4) | 10 (13.7) | ||
| PD | 4 (8.5) | 1 (2.8) | 0 | 2 (2.7) | ||
| NE | 0 | 0 | 0 | 0 | ||
| MRD (10−5)‡ | ||||||
| Patients evaluated, n | 50 | 38 | 72 | 75 | ||
| MRD negative, n (%) | 5 (10.0) | 0 | .0669§ | 20 (27.8) | 3 (4.0) | <.0001§ |
| . | CASTOR . | |||||
|---|---|---|---|---|---|---|
| Early relapse (<24 mos) . | Late relapse (≥24 mos) . | |||||
| D-Vd . | Vd . | P . | D-Vd . | Vd . | P . | |
| Response, n (%)∗ | ||||||
| Patients evaluated, n | 47 | 36 | 72 | 73 | ||
| ORR | 39 (83.0) | 26 (72.2) | .2415† | 70 (97.2) | 55 (75.3) | .0001† |
| ≥CR | 13 (27.7) | 4 (11.1) | .0657† | 39 (54.2) | 12 (16.4) | <.0001† |
| sCR | 2 (4.3) | 0 | 16 (22.2) | 5 (6.8) | ||
| CR | 11 (23.4) | 4 (11.1) | 23 (31.9) | 7 (9.6) | ||
| ≥VGPR | 31 (66.0) | 12 (33.3) | .0034† | 60 (83.3) | 34 (46.6) | <.0001† |
| VGPR | 18 (38.3) | 8 (22.2) | 21 (29.2) | 22 (30.1) | ||
| PR | 8 (17.0) | 14 (38.9) | 10 (13.9) | 21 (28.8) | ||
| MR | 2 (4.3) | 2 (5.6) | 1 (1.4) | 6 (8.2) | ||
| SD | 2 (4.3) | 7 (19.4) | 1 (1.4) | 10 (13.7) | ||
| PD | 4 (8.5) | 1 (2.8) | 0 | 2 (2.7) | ||
| NE | 0 | 0 | 0 | 0 | ||
| MRD (10−5)‡ | ||||||
| Patients evaluated, n | 50 | 38 | 72 | 75 | ||
| MRD negative, n (%) | 5 (10.0) | 0 | .0669§ | 20 (27.8) | 3 (4.0) | <.0001§ |
| . | POLLUX . | |||||
|---|---|---|---|---|---|---|
| Early relapse (<24 mos) . | Late relapse (≥24 mos) . | |||||
| D-Rd . | Rd . | P . | D-Rd . | Rd . | P . | |
| Response, n (%)∗ | ||||||
| Patients evaluated, n | 74 | 74 | 73 | 68 | ||
| ORR | 71 (95.9) | 55 (74.3) | .0002† | 66 (90.4) | 59 (86.8) | .4967† |
| ≥CR | 43 (58.1) | 11 (14.9) | <.0001† | 44 (60.3) | 30 (44.1) | .0558† |
| sCR | 18 (24.3) | 2 (2.7) | 28 (38.4) | 16 (23.5) | ||
| CR | 25 (33.8) | 9 (12.2) | 16 (21.9) | 14 (20.6) | ||
| ≥VGPR | 63 (85.1) | 31 (41.9) | <.0001‡ | 54 (74.0) | 49 (72.1) | .7987‡ |
| VGPR | 20 (27.0) | 20 (27.0) | 10 (13.7) | 19 (27.9) | ||
| PR | 8 (10.8) | 24 (32.4) | 12 (16.4) | 10 (14.7) | ||
| MR | 1 (1.4) | 8 (10.8) | 1 (1.4) | 1 (1.5) | ||
| SD | 2 (2.7) | 8 (10.8) | 5 (6.8) | 8 (11.8) | ||
| PD | 0 | 2 (2.7) | 0 | 0 | ||
| NE | 0 | 1 (1.4) | 1 (1.4) | 0 | ||
| MRD (10−5)‡ | ||||||
| Patients evaluated, n | 75 | 77 | 74 | 69 | ||
| MRD negative, n (%) | 23 (30.7) | 3 (3.9) | <.0001§ | 26 (35.1) | 12 (17.4) | .0225§ |
| . | POLLUX . | |||||
|---|---|---|---|---|---|---|
| Early relapse (<24 mos) . | Late relapse (≥24 mos) . | |||||
| D-Rd . | Rd . | P . | D-Rd . | Rd . | P . | |
| Response, n (%)∗ | ||||||
| Patients evaluated, n | 74 | 74 | 73 | 68 | ||
| ORR | 71 (95.9) | 55 (74.3) | .0002† | 66 (90.4) | 59 (86.8) | .4967† |
| ≥CR | 43 (58.1) | 11 (14.9) | <.0001† | 44 (60.3) | 30 (44.1) | .0558† |
| sCR | 18 (24.3) | 2 (2.7) | 28 (38.4) | 16 (23.5) | ||
| CR | 25 (33.8) | 9 (12.2) | 16 (21.9) | 14 (20.6) | ||
| ≥VGPR | 63 (85.1) | 31 (41.9) | <.0001‡ | 54 (74.0) | 49 (72.1) | .7987‡ |
| VGPR | 20 (27.0) | 20 (27.0) | 10 (13.7) | 19 (27.9) | ||
| PR | 8 (10.8) | 24 (32.4) | 12 (16.4) | 10 (14.7) | ||
| MR | 1 (1.4) | 8 (10.8) | 1 (1.4) | 1 (1.5) | ||
| SD | 2 (2.7) | 8 (10.8) | 5 (6.8) | 8 (11.8) | ||
| PD | 0 | 2 (2.7) | 0 | 0 | ||
| NE | 0 | 1 (1.4) | 1 (1.4) | 0 | ||
| MRD (10−5)‡ | ||||||
| Patients evaluated, n | 75 | 77 | 74 | 69 | ||
| MRD negative, n (%) | 23 (30.7) | 3 (3.9) | <.0001§ | 26 (35.1) | 12 (17.4) | .0225§ |
The early-relapse subgroup included patients with 1 prior line of therapy who progressed or relapsed <24 months after initiating their first line of therapy; the late-relapse subgroup included patients with 1 prior line of therapy who progressed or relapsed ≥24 months after initiating their first line of therapy.
ITT, intent-to-treat; MR, minimal response; NE, not evaluable; PD, progressive disease; PR, partial response; sCR, stringent complete response; SD, stable disease.
Response-evaluable population with 1 prior line of therapy. The response-evaluable population was defined as patients with a confirmed diagnosis of MM and measurable disease at the baseline or screening visit who received ≥1 administration of study treatment and had ≥1 postbaseline disease assessment.
P value was calculated using the Cochran-Mantel-Haenszel χ2 test.
ITT population with 1 prior line of therapy.
P value was calculated using the Fisher exact test.
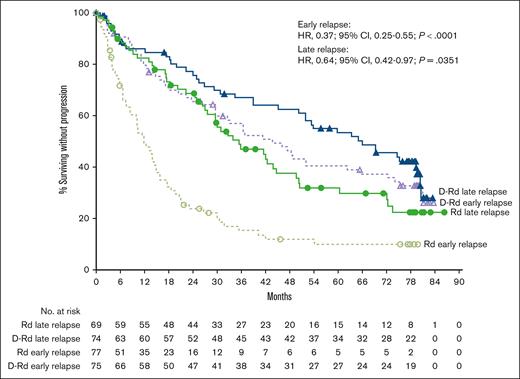
In the POLLUX study, the median PFS with D-Rd vs Rd was 43.7 vs 11.8 months (HR, 0.37; 95% CI, 0.25-0.55; P < .0001) in the early-relapse subgroup (<24 months) and 66.0 vs 35.5 months (HR, 0.64; 95% CI, 0.42-0.97; P = .0351) in the late-relapse subgroup (≥24 months; Figure 3). Rates of ≥CR, ≥VGPR, and MRD negativity (10−5 sensitivity) were higher with D-Rd vs Rd in both the early-relapse (<24 months) and late-relapse (≥24 months) subgroups (Table 3). Efficacy with D-Rd vs Rd using the 18-month early-relapse cutoff was generally consistent with that observed using the 24-month early-relapse cutoff (supplemental Figure 3; supplemental Table 6).
PFS by relapse subgroup (<24 and ≥24 months) in POLLUX. Kaplan-Meier plot of PFS in patients with 1 prior line of therapy who progressed or relapsed early or late after initiation of first line of therapy. The early-relapse subgroup included ITT patients with 1 prior line of therapy who progressed or relapsed <24 months after initiating their first line of therapy; the late-relapse subgroup included ITT patients with 1 prior line of therapy who progressed or relapsed ≥24 months after initiating their first line of therapy.
PFS by relapse subgroup (<24 and ≥24 months) in POLLUX. Kaplan-Meier plot of PFS in patients with 1 prior line of therapy who progressed or relapsed early or late after initiation of first line of therapy. The early-relapse subgroup included ITT patients with 1 prior line of therapy who progressed or relapsed <24 months after initiating their first line of therapy; the late-relapse subgroup included ITT patients with 1 prior line of therapy who progressed or relapsed ≥24 months after initiating their first line of therapy.
Discussion
Functional high-risk MM is becoming an increasingly more recognized subgroup of MM that is associated with poor outcomes. In a retrospective study of patients with MM treated with novel therapies (immunomodulatory drugs and proteasome inhibitors) at the Mayo Clinic between 2006 and 2014, the median OS was significantly shorter for patients with early relapse (within 12 months of starting initial therapy) vs for those with late relapse (21.0 months vs NR; P < .001).3 Outcomes were also evaluated in patients with early relapse after early autologous stem cell transplant (ASCT); the median OS from ASCT for patients relapsing within 12 months was 23.1 months compared with 122.2 months for the remaining patients (P < .001).3 Similarly, real-world data from a myeloma registry showed that median OS was markedly inferior for patients with early progression (within 12 months of commencing firstline therapy) vs those without early progression (20.2 vs 60.7 months; P < .001).5 These data highlight the need for effective therapies for this subgroup of patients with functional high-risk MM who relapse early after initial therapy.
Considering the poor prognosis of patients with functional high-risk MM, early identification of patients at risk of early relapse is crucial. In this post hoc analysis, few patients in the early-relapse subgroup (<24 months; Table 1) presented with high-risk cytogenetic factors (daratumumab, 22.6%; control, 22.6%) or ISS stage III disease (daratumumab, 14.4%; control,18.3%). Because only a subset of patients receive a second line of therapy and qualify for a clinical study, these patients may reflect the subgroup with higher risk disease. These results support the need for a broader definition of high-risk disease or stratification based on functional high-risk status in future studies.
This post hoc analysis evaluated the efficacy of daratumumab-containing regimens in the CASTOR and POLLUX studies in patient subgroups based on timing of progression or relapse (early or late) after initiation of the first line of therapy. Because of the limited number of patients who relapsed before 12 months, early relapse for this analysis was defined as patients with 1 prior line of therapy who progressed or relapsed <24 months (primary analysis) or <18 months (secondary analysis) after initiating their first line of therapy. Extending the cutoff for the early-relapse definition from <18 months to <24 months increases the sample size of the early-relapse group, providing greater statistical power to show differentiation between treatment groups. At a median follow-up of >6 years, PFS consistently favored the daratumumab-containing regimens across both the early- and late-relapse subgroups (24-month and 18-month cutoffs) in the pooled CASTOR/POLLUX population and for each study individually. In the CASTOR/POLLUX pooled data set, daratumumab reduced the risk of disease progression or death by 65% (HR, 0.35; 95% CI, 0.26-0.48; P < .0001) in the early-relapse (<24 months) subgroup and by 65% (HR, 0.35; 95% CI, 0.26-0.47; P < .0001) in the late-relapse (≥24 months) subgroup. Interestingly, the PFS benefit with daratumumab vs control was similar between the early- and late-relapse subgroups for the pooled analysis but was more pronounced with D-Vd vs Vd in the late-relapse subgroup in CASTOR and more pronounced with D-Rd vs Rd in the early-relapse subgroup in POLLUX. Small patient subgroups, particularly the CASTOR early-relapse subgroups, may limit the interpretation of results.
In the pooled analysis and for CASTOR and POLLUX alone, rates of ≥CR and MRD negativity were higher with the daratumumab-containing regimens vs control, regardless of relapse timing. OS favored the daratumumab-containing regimens in both the early and late-relapse subgroups in the pooled CASTOR/POLLUX population using the 24-month cutoff to define early progression or relapse; however, the difference in OS was not as pronounced between daratumumab and control for the early-relapse subgroup defined using the 18-month cutoff. Significant challenges are associated with the interpretation of OS data from this subgroup analysis and should be considered. There was low statistical power for OS analyses after dividing patients into small subgroups because of the relatively low number of OS events overall in each study. Therefore, OS data for the early-relapse and late-relapse subgroups are only reported for the CASTOR/POLLUX pooled population and not for each study individually.
A similar subgroup analysis of the phase 3 CANDOR study of daratumumab plus carfilzomib and dexamethasone (D-Kd) vs Kd alone for patients with RRMM based on the timing of relapse (early vs late) was previously conducted.32 Among patients in CANDOR who had received 1 prior line of therapy, ORR and ≥CR rates were higher in the D-Kd group than in the Kd group, regardless of relapse status (with early relapse defined as relapse <18 months after the first line of therapy), and PFS results also favored the D-Kd group, regardless of relapse status.32 A subgroup analysis of the phase 3 IKEMA study of isatuximab plus Kd (Isa-Kd) vs Kd alone was conducted for patients with RRMM who experienced early vs late relapse, with early relapse defined as relapse <12 months from initiation of the most recent line of therapy for patients with ≥2 prior lines, relapse <18 months for patients with 1 prior line, or relapse <12 months from ASCT.33 In patients with either early or late relapse, Isa-Kd improved PFS and depth of response vs Kd alone. Two multicohort phase 2 studies are also evaluating chimeric antigen receptor T-cell therapy for patients with MM and early relapse after initial therapy. In cohort B of the phase 2 CARTITUDE-2 study, patients with 1 prior line of therapy (including a proteasome inhibitor and immunomodulatory drug) who had early progression (≤12 months after ASCT or ≤12 months after start of antimyeloma therapy for patients who did not undergo ASCT) achieved deep and durable responses with ciltacabtagene autoleucel, with an 18-month PFS rate of 83%.34 In cohort 2a of the phase 2 KarMMa-2 study, idecabtagene vicleucel demonstrated frequent, deep responses and a median PFS of 11.4 months in patients with MM who experienced early relapse, defined as disease progression within 18 months of initiation of frontline treatment that included induction, ASCT, and lenalidomide-based maintenance therapy.35
Because many of the patients in CASTOR and POLLUX likely received a proteasome inhibitor for a fixed duration as part of their frontline therapy, patients who progressed or relapsed within the shorter time frame (<18 months) were more likely to exhibit some degree of proteasome inhibitor resistance than those who progressed or relapsed within the longer time frame (<24 months). Although the sample size was small for the early-relapse (<18 months) subgroup, the data from our analyses may suggest that, for patients who progressed or relapsed earlier (sooner after prior bortezomib exposure), secondline D-Rd may be the preferred treatment option for selected patients. Results from a secondary analysis of the ALLG MM21 study showed that patients with NDMM who were eligible for transplantation with suboptimal response or primary refractoriness to bortezomib induction who were switched to pre-ASCT D-Rd induction followed by post-ASCT D-Rd consolidation achieved substantial disease control.36 Furthermore, our finding that PFS with Vd was similar for patients with early relapse and late relapse further supports the possible role of proteasome inhibitor resistance and could be interpreted to indicate that Vd is not an appropriate secondline therapy for many patients in this setting. Thus, the data from our post hoc analyses could have implications on subsequent treatment selection based on the timing of progression or relapse.
Clinical data show that receipt of frontline daratumumab is important to maximize the duration of response to treatment. Results from the phase 3 ALCYONE and MAIA studies demonstrate significantly prolonged OS and PFS with the addition of daratumumab to standard-of-care regimens vs standard of care alone for transplant-ineligible patients with NDMM.18,20 Patients who do not receive frontline daratumumab and relapse after initial therapy can be salvaged with daratumumab-based regimens. As discussed, in CASTOR and POLLUX, D-Vd or D-Rd prolonged PFS, regardless of cytogenetic risk, and achieved higher rates of ≥CR and MRD negativity vs Vd or Rd alone for patients with RRMM.21,23 In CASTOR, the most pronounced PFS and OS benefit with D-Vd vs Vd was in patients with 1 prior line of therapy.21,22 In POLLUX, when analyzed based on the number of prior lines of therapy, patients with 1 prior line of therapy had the longest median PFS and OS.23,24 Moreover, with extended follow-up, no new safety concerns outside of the known safety profile of daratumumab have been observed.21-24
The current post hoc subgroup analyses showed PFS, OS, and depth of response benefits of daratumumab-containing regimens for patients with 1 prior line of therapy, regardless of progression/relapse timing (early or late), supporting the use of D-Vd and D-Rd for patients with RRMM, including those considered to have functional high-risk disease. However, even with the addition of daratumumab, patients with early relapse still have inferior outcomes compared with those who experience late relapse, which may provide rationale to add other agents to D-Vd or D-Rd using daratumumab as a backbone to further improve patient outcomes. It is also important to note that many patients with MM may not receive secondline therapy37,38 and that waiting until progression or relapse (whether early or late) to use daratumumab may not always lead to a significant survival benefit.
In summary, patients with functional high-risk MM who relapse early after initial therapy have poor outcomes, representing an unmet therapeutic need. At a median follow-up of >6 years, the results from these post hoc analyses showed PFS and depth of response benefits of daratumumab-containing regimens vs control for patients with 1 prior line of therapy, regardless of progression/relapse timing (early or late). Moreover, OS favored the daratumumab-containing regimens in both the early and late-relapse subgroups in the pooled CASTOR/POLLUX population. These results, combined with previous subgroup analyses of CASTOR and POLLUX,21,23 show that daratumumab-containing regimens maintain consistent clinical benefits for patients with high-risk RRMM, as defined either by cytogenetics or by early progression/relapse. Although daratumumab is unable to fully overcome the adverse prognosis of early relapse, our results support the use of D-Vd and D-Rd for patients with RRMM and 1 prior line of therapy, including for patients who progress or relapse early after initial therapy and are considered to have functional high-risk disease.
Acknowledgments
The authors thank the patients who participated in the CASTOR and POLLUX studies and their families as well as the study coinvestigators, research nurses, and coordinators at each of the clinical sites. Medical writing and editorial support were provided by Lisa Shannon of Lumanity Communications Inc and were funded by Janssen Global Services, LLC.
These studies (NCT02136134 and NCT02076009) were sponsored by Janssen Research & Development, LLC.
Authorship
Contribution: All authors participated in the conception and design of the study or in the acquisition, analysis, or interpretation of the data and in drafting, revising, and approving the final version of the manuscript before submission.
Conflict-of-interest disclosure: A.S. served on an advisory board for AbbVie, Amgen, Antegene, Bristol Myers Squibb, HaemaLogiX, Janssen, Pfizer, Roche, and Secura Bio; served on a speakers bureau for Bristol Myers Squibb and Janssen; received research support from AbbVie, Amgen, Bristol Myers Squibb, HaemaLogiX, Janssen, and PharmaMar; and received honoraria from AbbVie, Amgen, Antegene, Bristol Myers Squibb, HaemaLogiX, Janssen, Pfizer, Roche, and Secura Bio. P.M. served in a consulting or advisory role for Celgene, Janssen, Amgen, GlaxoSmithKline, Sanofi, AbbVie, Oncopeptides, and Roche; and received honoraria from Celgene, Janssen-Cilag, Amgen, GlaxoSmithKline, AbbVie, Sanofi, Oncopeptides, and Roche. M.-V.M. served in a consulting or advisory role for Takeda, Janssen-Cilag, Celgene, Amgen, AbbVie, GlaxoSmithKline, Pfizer, Regeneron, and Roche/Genentech, and received honoraria from Janssen-Cilag, Celgene, Amgen, Takeda, GlaxoSmithKline, AbbVie/Genentech, and Sanofi. H.G. received grants and/or provision of an investigational medicinal product from Amgen, Bristol Myers Squibb, Celgene, Chugai, Dietmar Hopp Foundation, Janssen, Johns Hopkins University, and Sanofi; received research support from Amgen, Bristol Myers Squibb, Celgene, Chugai, Janssen, Incyte, Molecular Partners, Merck Sharp & Dohme, Sanofi, Mundipharma GmbH, Takeda, and Novartis; served on advisory boards for Adaptive Biotechnology, Amgen, Bristol Myers Squibb, Celgene, Janssen, Sanofi, and Takeda; and received honoraria from Amgen, Bristol Myers Squibb, Celgene, Chugai, GlaxoSmithKline, Janssen, Novartis, and Sanofi. K.S. received honoraria from Celgene, Takeda, Ono Pharmaceutical, Amgen, Novartis, Sanofi, Bristol Myers Squibb, AbbVie, and Janssen; served in a consulting or advisory role for Amgen, Janssen, Takeda, and Celgene; and received research funding from Bristol Myers Squibb and Celgene. M.-D.L. received travel support from Takeda and Janssen, and received honoraria from AbbVie, Celgene, Janssen, and Takeda. P.S. received research support from Amgen, Celgene, Janssen, Karyopharm, SkylineDx, and Takeda, and received honoraria from and served on an advisory board for Amgen, Bristol Myers Squibb, Celgene, Janssen, Karyopharm, SkylineDx, and Takeda. R.Z.O. served on advisory committees for and received honoraria from AbbVie, Amgen, AstraZeneca, Biotheryx, Bristol Myers Squibb, Celgene, EcoR1 Capital, Forma Therapeutics, Genzyme, GlaxoSmithKline, Ionis Pharmaceuticals, Janssen, Juno Therapeutics, Karyopharm, Kite Pharma, Legend Biotech, Meridian Therapeutics, Monte Rosa Therapeutics, Molecular Partners, Neoleukin Therapeutics, Oncopeptides, Regeneron, Sanofi-Aventis, Servier, and Takeda; and received research funding from Asylia Therapeutics, Inc, Biotheryx, CARsgen Therapeutics, Celgene, Exelixis, Janssen, Sanofi-Aventis, and Takeda. S.-S.Y. received honoraria from Novartis; served on advisory boards for Amgen, Antengene, Astellas, Celgene, Janssen, Novartis, and Takeda; and received research funding from Kyowa Hakko Kirin, Roche-Genentech, and Yuhan Pharma. S.Z.U. received research funding from Amgen, Array BioPharma, Bristol Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Merck, Pharmacyclics, Sanofi, Seattle Genetics, SkylineDx, and Takeda; served in a consulting role for AbbVie, Amgen, Bristol Myers Squibb, Celgene, Edo Pharma, Genentech, Gilead, GlaxoSmithKline, Janssen, Oncopeptides, Sanofi, Seattle Genetics, Secura Bio, SkylineDx, Takeda, and TeneoBio; and served as a speaker for Amgen, Bristol Myers Squibb, Janssen, and Sanofi. K.W. received research support from Amgen, Bristol Myers Squibb/Celgene, Janssen, and Sanofi; received honoraria from AbbVie, Adaptive Biotech, Amgen, AstraZeneca, Bristol Myers Squibb/Celgene, BeiGene, GlaxoSmithKline, Janssen, Karyopharm, Novartis, Oncopeptides, Pfizer, Roche, Sanofi, Stemline, and Takeda; and received consulting fees from AbbVie, Adaptive Biotech, Amgen, Bristol Myers Squibb/Celgene, BeiGene, Janssen, GlaxoSmithKline, Karyopharm, Oncopeptides, Pfizer, Roche, Sanofi, and Takeda. D.R. received honoraria from Amgen, Bristol Myers Squibb/Celgene, GlaxoSmithKline, Janssen, and Takeda; served in a consulting or advisory role for Amgen, Bristol Myers Squibb/Celgene, Janssen, Karyopharm, and Takeda; received research funding from Bristol Myers Squibb/Celgene, Janssen, Millennium, and Takeda; provided expert testimony for Bristol Myers Squibb/Celgene and Janssen; and serves as the voluntary chief medical officer of the Canadian Myeloma Research Network. T.A. is an employee of and owns stock and options in Genmab. H.P., X.G., and J.C. are employees of Janssen and own stock in Johnson & Johnson. W.G.M. and R.C. are employees of Janssen. M.A.D. received honoraria from Amgen, BeiGene, Bristol Myers Squibb, Janssen, and Takeda. J.B.B. declares no competing financial interests.
Correspondence: Andrew Spencer, Malignant Haematology and Stem Cell Transplantation Service, Alfred Health-Monash University, 55 Commercial Rd, Melbourne, VIC 3004, Australia; email: andrew.spencer@monash.edu.
References
Author notes
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency.
Qualified researchers may request access through Yale Open Data Access Project site at http://yoda.yale.edu.
The full-text version of this article contains a data supplement.