TO THE EDITOR:
Sickle cell anemia (SCA) considerably modifies kidney structure and function.1 In SCA, the alterations of kidney function begin at very young stages of life with abnormally increased glomerular filtration rate,2 followed by albuminuria during childhood.3 Albuminuria has been associated with poor prognosis of individuals with SCA,4 whereas chronic kidney disease (CKD) has been associated with higher morbidity and early mortality.5-7 Albuminuria is common in individuals with SCA and predicts the progression of CKD.1 However, albuminuria is often intermittent in these individuals,8 and its progression over time needs to be better understood. Recently, Kasztan et al9 reported the natural history of albuminuria in the Birmingham Pediatric Sickle Cell Kidney Disease cohort. The aim of this study was to investigate the progression of albuminuria and its risk factors in a large prospective cohort of children and adolescents with SCA in a newborn cohort from Minas Gerais, Brazil. The protocol was approved by institutional review boards, and informed written consent was obtained. The detailed methods of the article are provided in supplemental Material. Of 555 participants included in the cohort,10 a total of 438 (78.9%) provided 2 samples and were included in the longitudinal analysis. Random spot urine specimens were collected during routine visits. Albuminuria was defined by urinary albumin-to-creatinine ratio (ACR) ≥ 30 mg/g. Participants were prospectively categorized based on the presence or absence of albuminuria at baseline and upon a follow-up urine test. Participants with albuminuria at baseline and at the follow-up urine test were considered to have persistent albuminuria (PA). Those without albuminuria at baseline and follow-up urine tests were classified as albuminuria absent or as having no albuminuria. Participants without albuminuria at baseline who developed albuminuria at the follow-up test and those with albuminuria at baseline but absence at follow-up were considered as having intermittent albuminuria. Baseline steady-state laboratory data were obtained from medical records. Baseline kidney injury biomarkers in the urine were measured using multiplex xMAP assays. Continuous variables were presented as mean ± standard deviation or median and interquartile range (IQR), whereas nominal variables were presented as frequencies. Multiple logistic regression was used to determine the association between covariables and PA adjusting for age. Participants with PA were compared with those with intermittent albuminuria and those without albuminuria. Statistical analyses were performed using the Statistical Package for the Social Science (SPSS) 26.0 (SPSS Inc, Chicago, IL) and GraphPad Prism 6 (GraphPad Software, La Jolla, CA) software, with significance defined as P < .05.
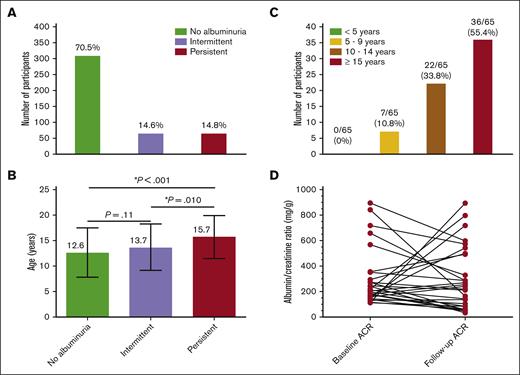
Out of 438 participants, 309 (70.5%) were classified as having no albuminuria, 64 (14.6%) as having intermittent albuminuria, and 65 (14.8%) as having PA (Figure 1A). Among participants with intermittent albuminuria, 38 (59.4%) had normal results at the first ACR measurement and abnormal results on the second measurement, whereas 26 (40.6%) had abnormal results at the first and normal results at the second ACR measurement. Mean age at baseline was 10.5 ± 4.7 years, and 229 (52.3%) were males. The median time from the fist ACR measurement to the follow-up was 2.3 years (range, 4.3 months to 6.4 years), providing 1192.3 patient-years. At follow-up urine test, participants with PA were significantly older (aged 15.7 ± 4.2 years) than participants with intermittent albuminuria (aged 13.7 ± 4.6 years; P = .01) and those without albuminuria (aged 12.6 ± 4.9 years; P < .001; Figure 1B). The prevalence of intermittent and PA found in this study conducted in Brazil as well as the progression of albuminuria over time were very similar to that reported in the United States.9 These findings suggest a comparable natural history of albuminuria in children with SCA living in a middle-income country and those living in developed countries. Environmental factors associated with pediatric sickle cell nephropathy are poorly characterized, but these data suggest a limited amount of phenotypic variability. Nevertheless, the burden of PA highlights the need for protocols and guidelines for early detection and management of this condition in individuals with SCA.
ACR status in a cohort of children and adolescents with SCA from Minas Gerais, Brazil. (A) Prevalence of intermittent and PA in the longitudinal analysis. (B) Age of participants by albuminuria category; each horizontal bar shows mean and standard deviation; statistically significant P values indicated by an asterisk (∗). (C) Frequency of participants with PA by categories of age at the confirmatory ACR measurement. (D) Variability from baseline to the follow-up ACR measurement in participants who had an ACR ≥ 100 mg/g at the enrollment. Three data points are outside the axis limits.
ACR status in a cohort of children and adolescents with SCA from Minas Gerais, Brazil. (A) Prevalence of intermittent and PA in the longitudinal analysis. (B) Age of participants by albuminuria category; each horizontal bar shows mean and standard deviation; statistically significant P values indicated by an asterisk (∗). (C) Frequency of participants with PA by categories of age at the confirmatory ACR measurement. (D) Variability from baseline to the follow-up ACR measurement in participants who had an ACR ≥ 100 mg/g at the enrollment. Three data points are outside the axis limits.
In this study, 314 participants (71.6%) were receiving disease-modifying therapy (hydroxyurea [HU], n = 255 [58.2%]; chronic blood transfusion, n = 8 [1.8%]; or both, n = 51 [11.6]). The median age at start of HU and transfusion therapies was 7.3 years (IQR, 4.7-10.2) and 5.5 years (IQR, 3.8-7.7), respectively. The median duration of HU and transfusion therapies at the time of baseline ACR measurement was 2.9 years (IQR, 1.5-5.1) and 5.4 years (IQR, 2.4-8.6), respectively. There was no association between disease-modifying therapy and PA (P = .72). Moreover, there was no difference in the variability of the follow-up ACR measurement by disease-modifying therapy (P = .58). Although this study was limited by its observational design and by the fact that adherence to HU was not systematically measured, the absence of association between disease-modifying therapy and PA was similar to that reported by Kasztan et al.9 Despite reports of HU preventing onset and progression of albuminuria,11 this aspect of SCA has not been studied in a randomized controlled trial (RCT) in pediatric patients. Recently, a systematic review identified a lack of adequately designed and powered RCTs on interventions to prevent or reduce kidney complications in individuals with SCA.12 Therefore, RCTs are required to evaluate the effects, especially long term, of new and established therapies on the progression of sickle cell nephropathy.
Eighteen individuals with SCA and PA (27.7%) developed an abnormal ACR before 10 years of age. Additionally, 7 individuals with PA (10.8%) developed two abnormal ACRs before 10 years of age (Figure 1C). Our findings reinforce the study by Kasztan et al,9 supporting the early screening for albuminuria, before 10 years of age. Our data provide evidence that future guidelines should specifically suggest ACR screening before the age of 10 years. The early detection of albuminuria is important to reduce morbidity and mortality due to CKD in individuals with SCA.
Thirty-eight participants in our cohort had an initial ACR of ≥100 mg/g and then a subsequent ACR test. The median ACR at the first episode of ACR ≥ 100 mg/g and at the next measurement was 195.2 mg/g (IQR, 135.3-308.5) and 153.4 mg/g (IQR, 59.1-495.6), respectively (Figure 1D). Overall, in 13 participants (34.2%), the ACR increased (median increase, 197.1%) by their next ACR measurements, whereas in 25 participants (65.8%), it had decreased (median decrease, 60.1%) by their next ACR measurement. Twenty-three (60.5%) participants maintained an ACR ≥ 100 mg/g, 10 (26.3%) had an ACR of between 30 and 100 mg/g, and 5 (13.2%) had normal ACR at their second ACR measurement. In comparison with the study of Kasztan et al,9 we found a larger percentage ACR change over time. This finding can be explained by the longer time between ACR measurements (2.2 years; IQR, 4.3 months to 5.0 years). Despite this, our data on the natural history of albuminuria variability after an ACR ≥ 100 mg/g are similar to those reported by Kasztan et al,9 reinforcing the need of end points in RCT that take into account the natural variability of ACR over time.
An ACR ≥ 100mg/g at baseline was associated with 75.9-times (95% confidence interval, 27.7-202.8; P < .001) higher odds of presenting PA, confirming that an ACR ≥ 100mg/g is a surrogate marker of PA.8,9 Although the use of random urine specimens may affect urine ACR variability, this threshold may allow early identification of individuals at the highest risk for CKD development, enabling early therapeutic interventions to prevent the decline of renal function.
Univariate analysis of risk factors of PA is described in supplemental Table 1. In multivariate analysis, participants with PA had lower hemoglobin and higher tissue inhibitor of matrix metalloproteinase-1 (TIMP-1) levels than the rest of participants (Table 1). When participants with PA were compared with those with absent albuminuria, the same model was obtained. This finding reinforces the influence of hemoglobin in the pathophysiology of PA.8,11 A meta-analysis has shown a reduction of 53% in the risk for albuminuria with improvements in hemoglobin levels of ≥1 g/dL.13 Additionally, hemolysis has been associated with endothelial dysfunction, inflammation, oxidative stress, and hypercoagulability.14 All these processes take part in the pathophysiology of albuminuria in individuals with SCA.15 TIMP-1 has role in extracellular matrix remodeling by controlling the activity of matrix metalloproteinases. Additionally, TIMP-1 modulates biological processes including fibrosis and apoptosis in the pediatric population with CKD.16 The association of PA with lower hemoglobin and increased TIMP-1 levels shed light on the potential therapeutic benefits of targeting these pathways.
In summary, this study supports data reported by Kasztan et al9 that suggest that the screening for albuminuria in children with SCA before the age of 10 years allows early identification of patients with albuminuria. Our data confirm that there is a natural variability in ARC measurements over time, which can be confounded by the efficacy of therapies in RCTs. Additionally, an ACR threshold ≥ 100 mg/g and lower levels of hemoglobin at baseline were associated with an increased risk of PA. Finally, we found to our knowledge, for the first time, the association of higher levels of TIMP-1 with PA.
Acknowledgments: The authors acknowledge all participants and parents of pediatric participants for their cooperation in being part of this study.
This work was supported by a grant from the Fundação de Amparo à Pesquisa de Minas Gerais (APQ-00131-17 and APQ-04261-17).
Contribution: A.R.B. designed the study, collected the data, performed experimental assays, and wrote the first draft of the manuscript; J.d.A.A. helped in data collection and performed sample preparation/experimental assays; A.C.S.-S. designed and coordinated the study and prepared the final revision of the manuscript; and all authors approved the final version of the manuscript and the submission for publication.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: André Rolim Belisário, Centro de Tecidos Biológicos de Minas Gerais, Fundação Hemominas, Rua das Goiabeiras 779, Lagoa Santa, 33400-000, Brazil; email: andrebelisario@yahoo.com.br.
References
Author notes
Data are available on request from the corresponding author, André Rolim Belisário (andrebelisario@yahoo.com.br).
The full-text version of this article contains a data supplement.