Visual Abstract
Chimeric antigen receptor (CAR) T-cell therapies have shown significant benefits in the treatment of hematologic malignancies, such as B-cell acute lymphoblastic leukemia (B-ALL) and B-cell lymphoma. Despite the therapeutic advances offered by these innovative treatments, failures are still observed in 15% to 40% of patients with B-ALL and >50% of patients with B-cell lymphoma. Several hypotheses have emerged including CD19-negative or -positive relapses, low CAR T-cell activation and/or expansion in vivo, or T-cell exhaustion. To date, in the European Union, CAR T cells granted with marketing authorization are autologous and thus associated with a strong heterogeneity between products. Indeed, the manufacturing of a single batch requires cellular starting material collection by apheresis for each patient, with variable cellular composition, and then challenging pharmaceutical companies to standardize as much as possible the production process. In addition, these cost and time-consuming therapies are associated with a risk of manufacturing failure reaching 25%. Thus, there is a growing need to identify early risk factors of unsuccessful production and/or therapeutic escape. Quality of the apheresis product, pathology progression, as well as previous treatments have been reported as predictive factors of the variability in clinical response. The aim of this review is to report and discuss predictive factors that could help to anticipate the manufacturing success and clinical response.
Introduction
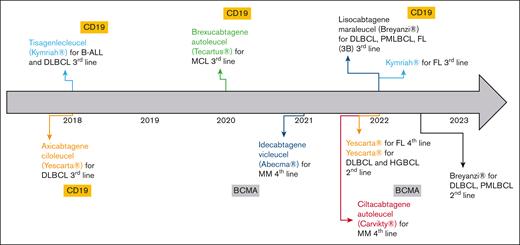
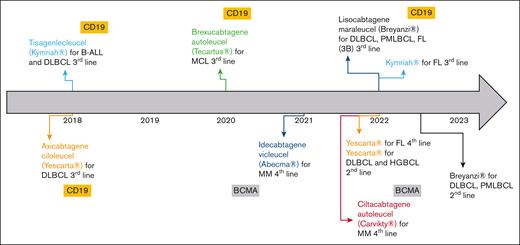
In the European Union, several advanced therapy medicinal products were granted with marketing authorization during the last decades. Despite several withdrawals from use, 18 advanced therapy medicinal products are still authorized as of January 2023.1 Gene therapies are the most represented (68%), especially since the emergence of chimeric antigen receptors (CAR) T cells. First authorized CAR T cells were tisagenlecleucel (tisa-cel, Kymriah) and axicabtagene ciloleucel (axi-cel, Yescarta) in 2018.2,3 To date, 6 CAR T-cell products have been authorized, and indications of tisa-cel and axi-cel have been extended (Figure 1). All commercial CAR T cells in the European Union are autologous, of second generation, and indicated in B-cell hematologic malignancies. Initially, CAR T cells were licensed to treat refractory or relapsed (R/R) pathologies after at least 2 lines of conventional treatment. Encouraging results of recent clinical trials aim at introducing CAR T cells earlier in the therapeutic strategy. In 2022, axi-cel was granted marketing authorization for the treatment of R/R diffuse large B-cell lymphoma (DLBCL) and high-grade BCL after firstline chemoimmunotherapy.3
Overview of CAR T cells granted with marketing authorizations (MA) in the European Union by January 2023, including extensions of their MA. BCMA, B-cell maturation antigen; FL, follicular lymphoma; HGBCL, high-grade B-cell lymphoma; MCL, mantle cell lymphoma; MM, multiple myeloma; PMBCL, primary mediastinal B-cell lymphoma.
Overview of CAR T cells granted with marketing authorizations (MA) in the European Union by January 2023, including extensions of their MA. BCMA, B-cell maturation antigen; FL, follicular lymphoma; HGBCL, high-grade B-cell lymphoma; MCL, mantle cell lymphoma; MM, multiple myeloma; PMBCL, primary mediastinal B-cell lymphoma.
However, lack of efficiency of these new treatments is still observed in 15% to 40% of patients with B-cell acute lymphoblastic leukemia (B-ALL) and > 50% of patients BCL.4-7 In addition, unsuccessful manufacturing can reach 7% for patients with B-ALL and 25% for those with non-Hodgkin lymphoma (NHL), resulting in significant delay of treatment.8,9 Manufacturing of autologous CAR T cells represents a challenge with high cost, waiting time, and difficulty to establish reproducible processes. Indeed, similar to any autologous cell–based therapy, CAR T cells require the manufacturing of a single batch with collection of cellular starting material by apheresis for each patient.10 This results in a heterogeneous starting cellular product with variable cell-population composition and/or fitness.10 As an example, it has been shown that monocyte rate in the apheresis product is disease dependent.8 Moreover, we recently reported significant differences in T-cell phenotype in cell collections for patients affected by the same disease.11 Taking together, these data highlight that the complete success of CAR T-cell therapies is related to intrinsic parameters including patient condition and extrinsic factors such as previous treatments. Indeed, the manufacturing of CAR T cells can result in 3 possibilities: (1) compliant manufacturing, meaning that CAR T cells were successfully produced, and the final product is compliant with specifications; (2) failure, when CAR T cells are not produced, and there is no final medicinal product; and (3) out of specifications, when CAR T cells are produced, but the final product is not compliant with specifications (ie, low dose or viability).
Thus, there is a real need to identify early predictive risk factors of unsuccessful manufacturing and/or therapeutic response. In this review, we reported the already identified clinical and/or biological parameters that could help to better anticipate the manufacturing success and/or clinical response to CAR T-cell therapies.
Patient disease
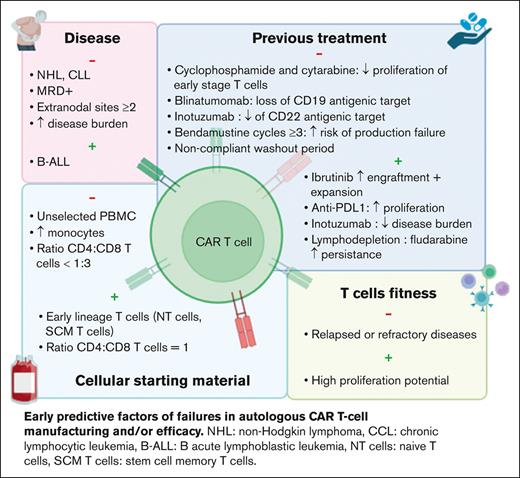
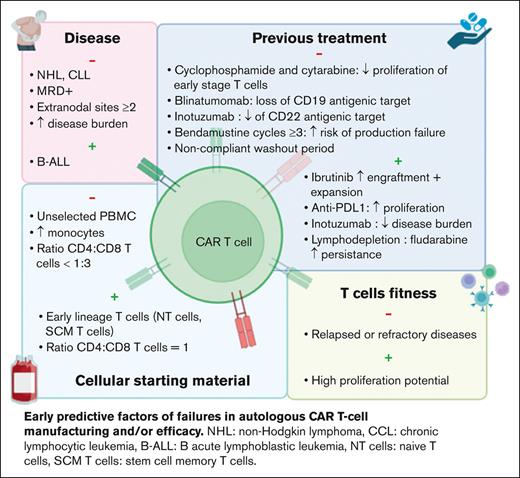
Several studies showed differences in clinical response to the same CAR T-cell drug when used for different pathologies. Pasquini et al conducted a first real-world prospective study in pediatric and young adult patients with B-ALL (n = 255) and adult patients with NHL (n = 155) treated with commercial tisa-cel.12 The complete remission (CR), duration of response, and event-free survival were higher in B-ALL than in NHL (85.5% vs 39.5%; 60.9% vs 55.3%; and 52.4% vs 38.7%, respectively). Another study showed a much lower efficacy in R/R chronic lymphocytic leukemia (CLL), with CR ≤30%.13 These results highlighted that the clinical efficacy of the same CAR T-cell medicinal product is disease related.
Several hypotheses were proposed to explain these differences. In B-ALL, CD19– relapse was associated with an increased tumor burden and detectable minimal residual disease before treatment.14 In BCL, CAR T-cell failure was correlated with tumor volume, extranodal sites ≥2, and a decreased expression of HLA-DR on monocytes.5,15
Disease progression can also influence CAR T-cell efficacy. Two pivotal studies (ZUMA-1 and JULIET) have evaluated the efficacy of axi-cel and tisa-cel in patients with DLBCL with an important heterogeneity in a patient’s eligibility and disease progression. JULIET study evaluated tisa-cel in patients with R/R disease after 2 lines of treatment, with progressing or bulky disease and chemotherapy bridging.7 Conversely, ZUMA-1 evaluated axi-cel in R/R within 12 months after autologous hematopoietic stem cell (HSC) transplantation, allowing only glucocorticoids bridging and excluding patients with disease progression.6 This resulted in significant differences in clinical responses with only 67% of infused patients and 40% CR in JULIET vs 91% and 54%, respectively, in ZUMA-1. These 2 studies were conducted independently, and no randomized clinical trial have compared both CAR T cells for the moment. In 2022, a real-word, retrospective, and matched comparison study analyzed the efficacy of axi-cel and tisa-cel in 809 patients with R/R DLBCL after ≥3 lines of treatment. Interestingly, this analysis confirmed a better efficacy of axi-cel. The CR and 1-year progression-free survival were 60% and 46.6%, respectively, for axi-cel compared with 42% and 33.2% for tisa-cel.16
Previous treatment
Inefficacy of autologous CAR T cells may be related to intrinsic defects of T cells due to previous therapies. Singh et al conducted a prospective observational study in pediatric patients with B-ALL and NHL to evaluate the expansion of T cells after each cycle of chemotherapy.17 Total T-cell count was similar between both diseases at diagnosis and throughout chemotherapy regimens, whereas their proliferative capacities in vitro were higher in B-ALL. One of the hypotheses would be that patients with B-ALL undergo allogeneic HSC before CAR T-cell treatment, thus, T cells used to manufacture CAR T cells are originally collected from healthy donors.13 However, T-cell expansion decreased after cyclophosphamide and cytarabine treatments in both pathologies, with a selective depletion of naïve T-cell compartment. Comparable results were observed in solid tumors even if the rate of naïve T cells was lower before any treatment.18
More recently, Jo et al identified the use of bendamustine with ≥3 cycles and washout period <3 months as a high-risk factor of tisa-cel production failure.19
Immunotherapies have been also identified as a risk factor of CAR T-cell failure. In patients with B-ALL, treatment with blinatumomab before CAR T cells induced the loss of CD19 antigenic target, increasing disease relapse.14,20,21 The use of alemtuzumab in CLL or daratumumab in multiple myeloma may exacerbate T-cell exhaustion.22,23
Conversely, other treatments administered before CAR T cells could have a positive impact. In preclinical studies, ibrutinib improved the engraftment and efficacy of anti-CD19 CAR T cells.24,25 Indeed, long-term treatment or cumulative cycles with ibrutinib may reverse T-cell dysfunction and increase CAR T-cell expansion.25 Thus, ibrutinib treatment before apheresis can be a perspective to improve CAR T-cell processing and clinical response. Immunomodulatory agents emerged as another strategy to improve CAR T-cell efficacy. Checkpoint inhibitors can act in synergy with CAR T cells,26 whereas cytokines such as interleukin-12 can overcome the tumor microenvironment.27 Inotuzumab, an anti-CD22 antibody reduces tumor burden and thus may be used before CAR T-cell administration.14 It is to be noted that disease burden is inversely correlated to CAR T-cell efficacy, however, it is associated with a reduced expression of antigenic target and thus may delay CAR T-cell activation and expansion in vivo.28
Finally, lymphodepletion performed few days before CAR T-cell administration is another key factor. Persistence of CAR T cells in vivo and clinical response are improved by the use of cyclophosphamide and fludarabine. Indeed, it allows for producing cytokines such as MCP-1 and interleukin-7 and modifying the tumor microenvironment, thus increasing biodistribution and activation of CAR T cells.29,30 High-dose lymphodepletive regimens may improve these parameters. In addition, lymphodepletion stimulates endogenous immune cells of patients but also of the administrated CD8+ and CD4+ T cells.31
Cellular starting material and T-cell fitness
To date, the majority of CAR T cells used in clinical practice are autologous, resulting in an important patient-dependent heterogeneity of the collected cellular starting material. Patients receive several chemo and/or immunotherapies before CAR T cells, thus decreasing peripheral blood T-cell number and increasing the risk of failure to reach the targeted number of CD3+ cells in up to 20% of apheresis.32 Several strategies have been evaluated to improve the yield of autologous T-cell harvest. O’Reilly et al designed a predictive model including the precollection CD3+ cell count and hematocrit and the processed blood volume.33 However, the use of unsorted peripheral blood mononuclear cells with random and variable compositions between patients may decrease manufacturing yield and antitumor activity. Indeed, processing of unselected cells generate a final product mainly composed of central memory and effector T cells.34 In addition, high contamination by monocytes has been shown to reduce T-cell transduction and CAR T-cell expansion in vitro, due to phagocytosis of magnetic beads used during T-cell isolation and/or activation.8,35 This can lead to the production of low doses of CAR T cells, resulting in significant clinical impact as shown in a real-world retrospective study of pediatric patients with B-ALL.36 OS, event-free survival, and RFS were decreased in patients treated with low doses of tisa-cel.
To increase T-cell expansion during the production process, Wang et al suggested to deplete monocytes in apheresis product containing ≥40% of CD14+ cells.8 Although monocyte depletion increases expansion in vitro, a positive selection of CD4+ and CD8+ T cells was shown to enhance CAR T-cell functionality in vivo, allowing for dose reduction.37 Moreover, a specific selection of defined CD4+ and CD8+ subsets could be a new approach to improve the manufacturing and clinical efficacy of autologous CAR T cells.10 Human CAR T cells derived from CD8+ central memory (CM) T cells and CD4+ naïve T cells in equal doses resulted in the highest antitumor effect in a mouse model.10 In clinical practice, the use of a defined CD8+:CD4+ ratio in patients with B-ALL allowed for increasing CAR T-cell expansion in vivo, offering the perspective of dose reduction.29 More recently, a phase 1/2 study evaluated liso-cel, an anti-CD19 CAR T cell with CD8+:CD4+ cells in equal doses (50 × 106:50 × 106) in CCL and R/R small lymphocytic lymphoma, resulting in 18% CR, compared with historic 0% to 5% CR.38 It is worth noting that CD4:CD8 ratio <1:3 was identified as a risk factor of tisa-cel production failure.19 This ratio may be affected by high quantities of monocytes, erythrocytes, or neutrophils in the apheresis product. Elavia et al showed that the contamination of cellular starting material by myeloid or erythroid cells were associated with higher quantities of CD4+ T cells, whereas CD8+ T cells were increased with neutrophils.39
Besides cell count, failure of CAR T-cell manufacturing has been related to intrinsic defects of collected T-cells with limited in vitro expansion, which is a key step to produce these therapies and a predictive factor of their in vivo proliferation and activity, that are directly associated with clinical outcomes. Ayuk et al have shown that poor axi-cel expansion in vivo in patients with BCL resulted in a shorter progression-free survival.40 They suggested to establish a cutoff of peak expansion in vivo to predict the clinical response. However, functional differences have been identified between T-cell subsets, resulting in variable expansion capacities.41 Studies in mouse models suggested that production of CAR T cells from naïve T cells, stem cells memory T cells, or CM T cells could be more efficient than from effector memory (EM) T cells or unsorted T cells.10,42 In clinic, a high level of naïve and SCM T cells was observed in patients with B-ALL and was associated with a higher expansion of T cells in vitro.17,18 However, T-cell functionality was altered by cumulative chemotherapy cycles, thus reducing their proliferative potential as previously described.17,18 In large BCL treated with axi-cel, a high rate of CCR7+CD45RA+ T cells (described as stem cell-like memory T cells) in apheresis products was associated with a high expansion of CAR T cells during cell processing and a better clinical response.43 Comparable results were observed in a real-world study including 30 patients with large BCL treated with tisa-cel.11 Interestingly, in CLL, CAR T-cell persistence in vivo and antitumor effect were observed in the presence of CM T cells.44,45 Altogether, these data suggested that CAR T-cell quality and efficacy could be improved by early collection of T cells, associated with sorting and enrichment of early stage T-cell subsets.46
A more recent approach to reduce the patient-dependent variability is the use of allogeneic CAR T cells. This approach offers the possibility to manufacture off-the-shelf medicinal products, with several batches produced from the same cellular starting material. In addition, cellular products display better intrinsic quality and potency of T cells than autologous CAR T cells.
Feasibility and safety of allogeneic CAR T cells have been shown firstly in R/R B-ALL. Two phase 1 studies including 21 patients reported CR in 14 patients with 10 requiring an allogeneic HSC graft.47 More recently, a phase 1 clinical trial evaluated safety, expansion, persistence, and activity of UCART123v1.2, an allogeneic CAR T-cell medicinal product that targets the CD123 antigen, in R/R acute myeloid leukemia. Sixteen patients received lymphodepletion with fludarabine and cyclophosphamide +/− alemtuzumab, followed by UCART123v1.2 dose escalation. Preliminary data showed a limited toxicity with only 1 grade 3 cytokine release syndrome and a proof of activity in 4 patients.48 Another phase 1 trial studied the UniCAR-T-CD123 in 14 patients with R/R acute myeloid leukemia. Early signs of activity were detected, with 3 CRs and 4 partial responses.49 Interestingly, both technologies were switchable on/off CAR T cells with the possibility to reverse rapidly the side effects. Indeed, in addition to the toxicities of autologous CAR T cells, graft-versus-host disease and T-cell rejection can be observed with allogeneic CAR T cells. Different strategies are investigated to limit allogeneic toxicities, including genetic modifications of HLA or the knockout of the endogenous T-cell receptor.
More recently, new technologies including the use of induced pluripotent stem cells to produce induced pluripotent stem cell–derived CAR T cells are investigated.50 This perspective appears promising to standardize the manufacturing but, at this day, are still preliminary to be compared with the efficiency of autologous CAR T cells.
Discussion
During the last decade, CAR T-cell medicinal products showed strongly encouraging clinical results in several hematologic malignancies including B-ALL, B-cell lymphoma, and multiple myeloma. The increase in the proportion of eligible patients, associated with better-controlled toxicities, is required to rapidly improve manufacturing capacities. However, several factors are still challenging CAR T-cell efficiency and manufacturing standardization that may affect treatment delay and/or failures. The automation of production processes has emerged as a promising strategy to harmonize processing of cell and gene therapies. However, interdonor/patient heterogeneity still represents a real challenge.
Identification of early predictive factors of processing and/or clinical failures will allow for improving the quality and efficacy of these treatments, helping with therapeutic decision-making as early as possible. Detection of T-cell subsets with high proliferative capacities, survival, functionality, and specificity to antigenic target will help improving the development of more efficient CAR T cells. Early lineage phenotypes have been identified as positive factors to enhance CAR T-cell activity. However, cumulative chemotherapy cycles, previous use of toxic treatments, as well as noncompliant washout period alter T-cell fitness and thus CAR T-cells efficiency. In addition, pre–CAR T-cell treatment may reduce the expression of antigenic target. Thus, detection of negative clones (ie, CD19– cells) in peripheral blood before apheresis may be used as an early predictive factor of patient eligibility. In addition, patient status, tumor burden, detectable minimal residual disease, and disease progression and/or aggressiveness can be used as early prognostic factors (before CAR T-cell manufacturing), whereas CAR T-cell expansion in vivo may predict the treatment outcome after CAR T-cell infusion.
To conclude, prescreening of patients should combine several factors including disease condition, previous treatments, and analysis of the apheresis product. Even better, identification of earlier predictive factors in peripheral blood will help in selecting patients before apheresis to avoid treatment delay and the manufacturing of these costly treatments.
Acknowledgment
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authorship
Contribution: C.B. prepared, created, and wrote the manuscript; J.L. supervised the manuscript; and M.M. wrote and supervised the manuscript.
Conflict-of-interest disclosure: J.L. and M.M. report consultancy fee from Kite/Gilead, Novartis, BMS, and Janssen. C.B. reports no competing financial interests.
Correspondence: Miryam Mebarki, Unité de Thérapie Cellulaire, INSERM U976 CIC BT Hôpital Saint-Louis, 1 Ave Claude Vellefaux, Paris 75010, France; Faculté de pharmacie, Université Paris Cité, 4 avenue de l’observatoire, Paris 75006, France; email: miryam.mebarki@aphp.fr.
References
Author notes
Data are available on request from the corresponding author, Miryam Mebarki (miryam.mebarki@aphp.fr).