In postpartum samples, a mechanism compensated for consumption of coagulation proteins and maintained peak thrombin output.
In the postpartum samples, CT-001 enhanced the hemostatic activity, supporting its potential as a therapeutic for postpartum hemorrhage.
Visual Abstract
The hemostatic system is upregulated to protect pregnant mothers from hemorrhage during childbirth. Studies of the details just before and after delivery, however, are lacking. Recombinant factor VIIa (rFVIIa) has recently been granted approval by the European Medicines Agency for the treatment of postpartum hemorrhage (PPH). A next-generation molecule, CT-001, is being developed as a potentially safer and more efficacious rFVIIa-based therapy. We sought to evaluate the peripartum hemostatic status of pregnant women and assess the ex vivo hemostatic activity of rFVIIa and CT-001 in peripartum blood samples. Pregnant women from 2 study sites were enrolled in this prospective observational study. Baseline blood samples were collected up to 3 days before delivery. Postdelivery samples were collected 45 (±15) minutes after delivery. Between the 2 time points, soluble fibrin monomer and D-dimer increased whereas tissue factor, FVIII, FV, and fibrinogen decreased. Interestingly, the postdelivery lag time and time to peak in the thrombin generation assay were shortened, and the peak thrombin generation capacity was maintained despite the reduced levels of coagulation proteins after delivery. Furthermore, both rFVIIa and CT-001 were effective in enhancing clotting activity of postdelivery samples in activated partial thromboplastin time, prothrombin time, thrombin generation, and viscoelastic hemostatic assays, with CT-001 demonstrating greater activity. In conclusion, despite apparent ongoing consumption of coagulation factors at the time of delivery, thrombin output was maintained. Both rFVIIa and CT-001 enhanced the upregulated hemostatic activity in postdelivery samples, and consistent with previous studies comparing CT-001 and rFVIIa in vitro and in in vivo, CT-001 demonstrated greater activity than rFVIIa.
Introduction
Progressive changes in the coagulation system occur over the course of pregnancy, labor, and delivery to provide enhanced hemostasis for pregnant women.1-9 Dysregulation of these concerted changes can lead to thrombosis and excessive bleeding at the time of delivery. Pregnancy is the leading cause of death among childbearing-aged women worldwide, and 25% of the estimated 295 000 women who die in childbirth each year die from postpartum hemorrhage (PPH).10 The overwhelming majority die in low-income countries, but an unacceptably high number also die in high-income countries.11-14 Many of the deaths occur in the setting of a coagulopathy that develops acutely when uterotonics and sutures have failed to control the usual obstetrical and surgical causes of PPH.
Massive obstetrical hemorrhage (severe PPH) with coagulopathy complicates ∼0.25% to 0.5% of deliveries.15-17 The common risk factors for severe PPH include hemolysis, elevated liver enzymes, and low platelets syndrome, surgical bleeding, placenta accreta, and uterine atony.18 However, the majority of PPH events are not associated with these factors and, thus, cannot be predicted. The treatment of severe PPH relies on the rapid activation of a massive transfusion protocol,19 but massive transfusion is resource intensive and not always successful. A randomized trial performed in mostly underresourced countries demonstrated the safety and possibly modest benefit of the antifibrinolytic medication tranexamic acid when administered within 3 hours of delivery,20 but the supplemental use of fibrinogen concentrate has not demonstrated significant benefit in PPH.21-23 Thus, the development of additional pharmacological interventions for severe PPH are needed. Recombinant activated factor VII (rFVIIa) has been used in the management of severe PPH unresponsive to any other therapy24-26 and has been recently granted approval by the European Medicines Agency. One of the drawbacks of rFVIIa, however, is its prothrombotic side effects.
CT-001, a novel FVIIa with rapid clearance, might ameliorate or eliminate this drawback of prothrombotic effects. CT-001 is engineered to be rapidly cleared from the circulation by having the addition of 4 desialylated N-glycans. Two of the desialylated glycans are at the native N-glycan sites and another 2 desialylated glycans are introduced by protein engineering at T106N and V253N. All glycans have had their terminal sialic acids removed to promote active clearance via asialoglycoprotein receptors. CT-001 is also engineered to have enhanced activity by having P10Q and K32E substitution introduced to its vitamin K–dependent carboxylation/γ-carboxyglutamic domain for enhanced phospholipid affinity and activity.27 In mouse studies, CT-001 demonstrated increased efficacy in reducing bleeding with significantly reduced thrombogenicity.28
In this study, we sought to determine the changes in the hemostasis system before delivery and within an hour after delivery, as well as to evaluate the ex vivo hemostatic response of postdelivery blood samples to rFVIIa and the novel procoagulant agent, CT-001.
Materials and methods
Study population
This was a prospective, observational study of hemostatic markers in women in the immediate postpartum period, approved by the institutional review boards at Duke University and Pusan National University Hospital (PNUH). Patients were approached regarding possible participation, either in the outpatient clinic or on presentation to the labor and delivery unit. Women at higher risk for PPH, such as patients with placenta accreta spectrum undergoing scheduled cesarean delivery, were preferentially enrolled. The inclusion criteria were aged ≥18 years; gestational age of ≥28 weeks, 0 days; English speaking at Duke University and Korean speaking at PNUH; able to provide own informed consent; and having an intention of being available for the entire study period and complete all relevant study procedures. The exclusion criteria were active labor at time of consent as defined as regular uterine contractions and cervical dilation of ≥4 cm, anticoagulation within 24 hours of admission to the hospital for delivery (low-dose aspirin was allowed), known bleeding disorder, thrombocytopenia < 100 000/μL, or any other condition that, in the opinion of the investigators, would pose a health risk to the patient or would otherwise interfere with the evaluation of the study objectives.
Study procedures
Patients who consented to participate had their blood drawn for baseline hemostatic studies before delivery and at 45 ± 15 minutes after the delivery of the infant. PPH was defined as ≥1000 mL for vaginal and cesarean delivery within 24 hours postpartum. If PPH occurred, specific information was obtained with respect to suspected underlying causes of PPH, timing of PPH, blood products administered, and hemostatic agents used. At Duke University, samples were transferred to the Duke Hemostasis and Thrombosis Center Core Laboratory for processing. Blood loss at delivery was quantified by Triton real-time blood loss monitoring system. Data were entered into a Research Electronic Data Capture (REDCap) database (developed by Vanderbilt University, TN). At PNUH, samples were transferred to the Laboratory of Diagnostic Hematology as well as the Trauma Center for processing. Blood loss was quantified by weighing pads. Data were entered into PNUH OBGYN Clinical Database system. At both sites, the electronic medical record of each participant was reviewed to obtain basic demographic, medical, and obstetric data such as maternal age, race/ethnicity, parity, gestational age, medical comorbidities, medications, clinical laboratory results, and mode of delivery.
Laboratory procedures
Hemoglobin, hematocrit, platelet counts, and viscoelastic assays were performed on site in the Hemostasis and Thrombosis Center Core Laboratory at Duke University and in the Laboratory of Diagnostic Hematology as well as the Trauma Center at PNUH. Plasma samples were sent to a central laboratory, MLM Medical Labs (Memphis, TN) for coagulation activity analysis. Samples shipped to the central laboratory did not contain any patient identifiers and contained only the participant ID and study time point.
Coagulation protein measurements in peripartum samples
Fibrinogen was measured by STA–Fibrinogen 5, FV by STA-Neoplastin with STA-Deficient V kit, FVIII by STA–PTT 5 kit with STA-Deficient VIII kit, FX by STA-Neoplastin with STA-Deficient X kit, protein C by Staclot protein C, D-dimer by STA-Liatest D-Di, and soluble fibrin monomer by STA-Liatest FM kit from Stago (Parsipanny, NJ) according to manufacturers’ protocols, on a Stago Compact Max system. Tissue factor (TF) was measured by Tissue Factor Quantikine enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN).
APTT and PT clotting assays
The effect of CT-001 and rFVIIa on citrated patient samples was evaluated by activated partial thromboplastin time (APTT) with STA-PTTA following manufacturer’s protocol. Prothrombin time (PT) was performed with STA-Neoplastine CI Plus 5 (rabbit brain thromboplastin). Both clotting assays were performed on a Stago Compact Max in duplicates.
TGA
Thrombin generation activity (TGA) in patient’s citrated plasma was measured in the presence of CT-001 or rFVIIa using MP reagent (containing 4 μM phospholipid) with a Fluoroskan Ascent reader (Stago) according to the manufacturer’s protocol.
Viscoelastic hemostatic assay by whole-blood ROTEM
CT-001 and rFVIIa variants (3.16-316 nM) were incubated in patient’s citrated whole blood. INTEM (ellagic acid and calcium chloride) or NATEM (calcium chloride) reagent was used as activator, and assays were performed on rotational thromboelastogram (ROTEM) machines following the manufacturer’s protocol (Werfen, Bedford, MA).
Statistical analysis
Statistical analyses were performed by Prism 9 (GraphPad Software, San Diego, CA).
Results
Patient baseline characteristics
During the study period, 18 patients were enrolled at Duke University, with data and samples available for 17; and 11 were enrolled at Pusan National University (Table 1). Although recruited from 2 sites of different ethnic compositions, the pregnant women had similar age, parity, and gestational age. Besides ethnicity differences, 6 of 11 patients enrolled at Pusan National University had total placenta previa, 5 of 11 patients with placenta accreta spectrum, and 10 of 11 underwent cesarean delivery, of which classical incision was carried out for 8 of the patients. At Duke University, 3 of 17 patients had placenta accreta spectrum, and only 2 classical incisions were performed for the 17 patients who underwent cesarean delivery. A difference in blood loss at delivery was observed between Duke University (508 mL [interquartile range [IQR], 332-641 mL]) and PNUH (1000 mL [IQR, 500-2000 mL]). Patients were divided into non-PPH and PPH groups. The blood counts and plasma coagulation protein levels (supplemental Table 1) as well as the coagulation activity parameters (supplemental Table 2) before and after delivery were compared within each group. In the non-PPH group, the majority of the parameters underwent statistically significant changes after delivery. In contrast, few parameters in the PPH group had statistically significant changes after delivery. This was likely contributed by the low number of patients with PPH who were enrolled. A comparison of the non-PPH and PPH samples for the before delivery and after delivery time points showed that among the 22 parameters analyzed, only 3 parameters (fibrinogen, TGA peak thrombin, and TGA velocity index) in the predelivery samples and 3 other parameters (FV, FX, and TGA time to peak) in the postdelivery samples showed statistical significance (supplemental Table 3). With the sparse number of parameters showing statistical significance, patients in the non-PPH and PPH groups were combined for statistical analyses (Tables 2 and 3).
Blood counts and plasma coagulation protein levels before and after delivery
Before delivery, patients had a median hemoglobin of 10.6 g/dL [IQR, 9.8-11.5 g/dL], a median hematocrit of 32.0 % [IQR, 29.7-35.2%], and a median platelet count of 192 × 109/L [IQR, 171-234 × 109/L]; Table 2). After delivery, an 8.5% decrease in hemoglobin (9.7 g/dL [IQR, 9.2-10.3 g/dL]; P < .0001) and a 6.2% decrease in hematocrit (30.0% [IQR, 28.5-30.6%]; P < .0001) were observed. There was also an 11.5% reduction in platelet count (170 × 109/L [IQR, 142-200 × 109/L]; P = .0001). Statistically significant changes in the concentration of clotting proteins involved were observed after delivery. An 11% reduction in plasma TF (P = .0007) and FX (P < .0001) was observed. The levels of other coagulation factors were also reduced, with a 13% reduction in FVIII (P < .018) and FV (P < .0001), and a reduction of 19% in fibrinogen (P < .0001). Protein C was reduced by 14% (P < .0001). An increase of 333% was observed for soluble fibrin monomer (P < .0001) and 119% for D-dimer (P < .0001).
Coagulation activity of plasma and whole-blood samples before and after delivery
Plasma clotting assays, TGA, and viscoelastic hemostatic assays (VHAs) were performed on patient samples (Table 3). The average APTT clotting time (CT) before and after delivery were similar. A slight prolongation in the average PT was observed after delivery (13.2 seconds [IQR, 13.0-13.9 seconds]) in comparison with before delivery (12.8 seconds [IQR, 12.6-13.4 seconds]). In TGA, similar peak thrombin measurements and interquartile ranges were observed before and after delivery. The velocity index also showed no significant difference. However, a 22% decrease in endogenous thrombin potential (ETP) was observed after delivery (P < .018). A 13% shortening of lag time (P < .0015) and time to peak (P < .0010) was observed after delivery. In VHAs performed using ROTEM, the α angle and the CT measurements in whole-blood samples activated by INTEM reagent showed no significant differences between samples obtained before and after delivery. The NATEM α-angle measurements also showed no significant change. However, when activated with NATEM reagent, CT was shorter in postdelivery samples (333 seconds [IQR, 250-429 seconds]) than in predelivery samples (401 seconds [IQR, 312-468 seconds]; P < .0052).
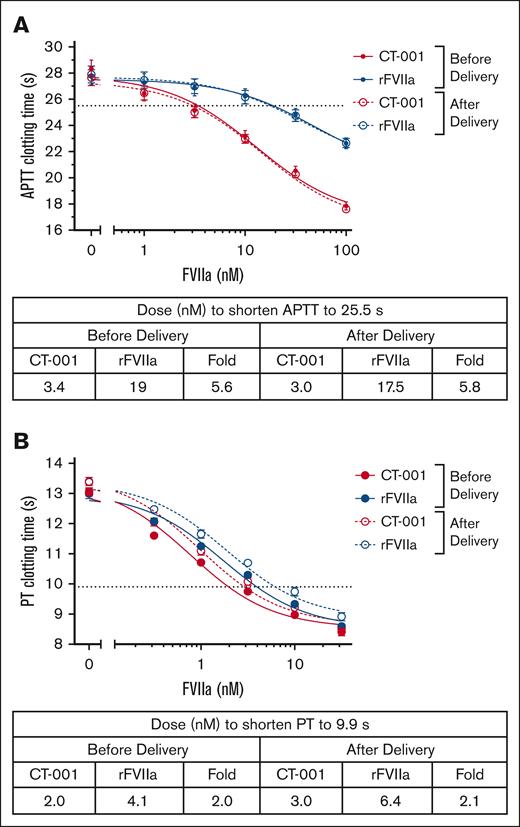
Potency of CT-001 and rFVIIa in plasma clotting assays
CT-001 has been reported to have a specific activity 4.5 times higher than rFVIIa.27 The plasma concentration after an administration of 60 to 90 μg/kg rFVIIa for PPH is ∼12 to 17 nM.24,29 Therefore, we expect 2.6 to 3.7 nM (average of 3 nM) CT-001 to be the likely plasma concentrations in humans for efficacy. Thus, the effect of 3 nM CT-001 on the postdelivery samples was chosen as the threshold for comparing CT-001 and rFVIIa treatment effect. In the APTT assay, rFVIIa provided a similar dose-dependent shortening of the CT to plasma samples from before delivery and after delivery. A shortening of CT to the 25.5-second threshold was achieved with ∼17.5- to 19-nM rFVIIa. In contrast, CT-001 had a potency 5.6 to 5.8 times higher than rFVIIa. Only ∼3 nM of CT-001 was needed to shorten APTT CT to the same level (Figure 1A; supplemental Figure 1). In the PT assay, plasma samples from after delivery were more sensitive to FVIIa activity. A shortening of CT to a 9.9-second threshold was achieved with ∼6.4 nM rFVIIa. CT-001 still had 2.1 times higher potency than rFVIIa. Only ∼3.0 nM CT-001 was needed to shorten PT CT to the same level (Figure 1B; supplemental Figure 2).
Effect of CT-001 and rFVIIa on clotting time of patient samples at baseline and 45 (±15) minutes after delivery time. (A) APTT. (B) PT.
Effect of CT-001 and rFVIIa on clotting time of patient samples at baseline and 45 (±15) minutes after delivery time. (A) APTT. (B) PT.
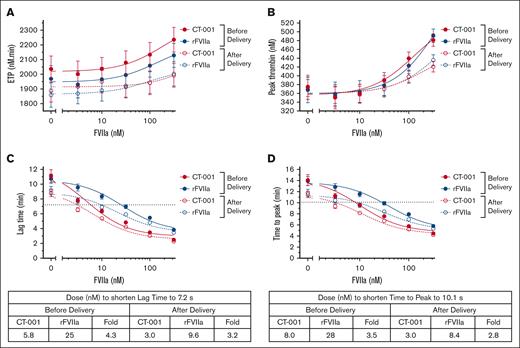
Potency of CT-001 and rFVIIa in TGA
In the TGA activated by MP reagent, no significant changes were observed in ETP with up to 316 nM of CT-001 or rFVIIa (Figure 2A). Minimal changes in peak thrombin generation were observed with 3.16 to 31.6 nM of CT-001 or rFVIIa. A ≥100 nM concentration of CT-001 or rFVIIa was needed to induce significant elevation in peak thrombin (Figure 2B). A dose-dependent shortening in the lag time of plasma samples was observed after treatment with CT-001 or rFVIIa (Figure 2C; supplemental Figure 3). Overall, CT-001 was threefold to fourfold more potent than rFVIIa in shortening the lag time. A similar shortening was observed for time-to-peak measurements. Plasma samples from after delivery were more sensitive to FVIIa activity, and CT-001 was threefold to fourfold more potent than rFVIIa (Figure 2D; supplemental Figure 4).
Effect of CT-001 and rFVIIa on TGA of patient samples at baseline and 45 (±15) minutes after delivery time. (A) ETP, (B) peak thrombin, (C) lag time, and (D) time to peak.
Effect of CT-001 and rFVIIa on TGA of patient samples at baseline and 45 (±15) minutes after delivery time. (A) ETP, (B) peak thrombin, (C) lag time, and (D) time to peak.
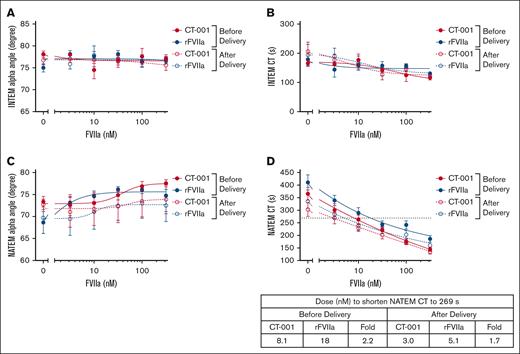
Potency of CT-001 and rFVIIa in VHA
Patient peripartum whole-blood samples activated by the INTEM reagent in the ROTEM assay had a narrow dynamic range relative to the high variation in sample measurements. The α angle for untreated samples was ∼77°. The addition of CT-001 or rFVIIa did not further increase the INTEM α angle (Figure 3A; supplemental Figure 5). The average CT for untreated postdelivery samples was ∼200 seconds. The high dose of 316 nM CT-001 or rFVIIa only shortened CT to an average of 117 and 123 seconds, respectively. Thus, the use of INTEM reagent was suboptimal for studying dose response of patient plasma to CT-001 and rFVIIa (Figure 3B; supplemental Figure 6). The NATEM α angle measurements were also not significantly changed in the presence of CT-001 or rFVIIa (Figure 3C; supplemental Figure 7). In contrast, the NATEM reagent provided a much wider dynamic range for detecting CT-001– and rFVIIa-mediated shortening of CT measurements (Figure 3D; supplemental Figure 8). Compared with whole-blood samples from delivery, samples from after delivery were more sensitive to FVIIa activity. Samples from before delivery required 8.1 nM CT-001 or 18 nM rFVIIa to reduce the CT to the threshold of 269 seconds. In contrast, samples from after delivery only required 3.0 nM CT-001 or 5.1 nM rFVIIa to reduce the CT to 269 seconds. In the NATEM assays, CT-001 was approximately twofold more potent than rFVIIa in patient samples.
Effect of CT-001 and rFVIIa on VHA α angle and CT measurements of patient samples at baseline and 45 (±15) minutes after delivery time. (A) INTEM α angle, (B) INTEM CT, (C) NATEM α angle, and (D) NATEM CT.
Effect of CT-001 and rFVIIa on VHA α angle and CT measurements of patient samples at baseline and 45 (±15) minutes after delivery time. (A) INTEM α angle, (B) INTEM CT, (C) NATEM α angle, and (D) NATEM CT.
Discussion
Changes in the hemostatic system serve to provide protection from hemorrhage during delivery. Observations about these observed changes have been made on samples from the different trimesters before delivery and some during the postpartum period.1-6,30 There is only a limited amount of literature on changes immediately before delivery and within an hour after delivery.7-9 In this study, we sought to expand our understanding of the hemostatic changes during this period of time by using patients’ paired samples. Based on coagulation assays and the coagulation factor measurements, the activation of the hemostatic system immediately after delivery was evident in patients within this study. Furthermore, in these postdelivery samples, rFVIIa and, to a greater extent, CT-001 demonstrated the potential of providing additional prohemostatic activity.
Patients in this study were recruited from Duke University and PNUH. Although the ethnic composition of the patients from the 2 sites were different, the pregnant women had similar age, parity, and gestational age. The higher amount of blood loss observed at PNUH could be because of the number of patients with total placenta previa (6 of 11 patients) as well as the number of cesarean deliveries (10 of 11 patients) of which classical incision was carried out in 8. At Duke University, there were 3 patients with placenta accreta spectrum, and, of the 17 patients who underwent cesarean delivery, 2 classical incisions were performed. Also, Duke University measured blood loss by the Triton automated quantification approach31 whereas PNUH used the gravimetric method, which could sometimes overestimate blood loss because of the hard-to-avoid mixing of amniotic fluid and irrigation fluids and their incorporation onto the surgical pads. Thus, the difference in blood loss measurements was likely contributed to by a combination of these aforementioned factors. After delivery, a decrease in hemoglobin, hematocrit, and platelet count, ranging from 6.2% to 11.5%, was observed. The decrease was modest and reflective of the biological process of delivery and peripartum support treatment. These observations are similar to results reported in a study with healthy patients8 and a study of patients with PPH.32
During delivery, an upregulation in coagulation was observed. Soluble fibrin monomer was increased by 3.3-fold and D-dimer by 1.2-fold postpartum whereas TF, FVIII, FV, FX, and fibrinogen were reduced by 11% to 19%, with ETP in the TGA reduced by 22%. Interestingly, the postdelivery lag time and time to peak in TGA were shortened although the procoagulant protein levels were decreased in patients. Additionally, the velocity index and peak thrombin generation capacity was maintained under these reduced levels of coagulation proteins. This preservation of velocity index and peak thrombin generation could potentially be contributed to by a downregulation in the natural anticoagulant pathways postpartum. This possibility is supported by the 14% reduction in protein C levels. In future studies, the levels of other natural anticoagulant proteins such as TF pathway inhibitor (TFPI), antithrombin, and protein S after delivery could also be monitored to elucidate the underlying mechanisms for the maintenance of peak thrombin generation. In fact, a recent study has shown elevated TFPI is associated with PPH.33 These dynamic changes in procoagulant and anticoagulant proteins and their regulatory control could represent a novel compensatory mechanism for maintaining thrombin output for hemostasis while there is an ongoing consumption of coagulation factors during the bleeding challenge of childbirth.
Although TGA and coagulation protein activity measurements together provided detailed monitoring of the relationships of thrombin generation and coagulation protein levels, the point-of-care VHAs represented a timelier approach for the monitoring of hemostatic changes. In the INTEM assays, a nonstatistically significant shortening in CT in postdelivery samples was observed. This lack of significance could perhaps be because of the low number of patients. It is worth noting that the lack of significant differences in EXTEM, INTEM, FIBTEM, and APTEM assays were also reported previously in a larger study.7 Interestingly, under reduced levels of coagulation proteins, a statistically significant shortening in CT in the postdelivery samples could be detected by NATEM assay. This observation provides another line of evidence of a compensatory mechanism for sustaining hemostasis in the presence of lowered coagulation protein levels.
The hemostatic changes observed in this study collectively indicated that upregulated coagulation existed before delivery and was further activated to support the delivery process. Therefore, a cautious recognition of the potential thromboembolic risk associated with the use of a procoagulant therapy for severe PPH remains. This is illustrated in a randomized trial in which rFVIIa was introduced in addition to standard of care for treatment of patient with severe PPH. In the aforementioned trial, rFVIIa demonstrated a highly statistically significant benefit in combination with standard of care, reducing the use of second-line therapies by 41%. However, it should be noted that 1 ovarian vein thrombosis and 1 deep vein thrombosis with a nonsevere pulmonary embolism were observed in the rFVIIa treatment group of 42 patients.24
A procoagulant molecule with a short half-life in circulation could potentially minimize the risk of thrombogenicity in patients with severe PPH while maintaining therapeutic benefit. CT-001 is a novel FVIIa, engineered to have enhanced activity while being rapidly cleared in an attempt to reduce thrombogenicity.27,28 CT-001 provided enhanced coagulation activity compared with rFVIIa, with APTT shortened by 5.8-fold and PT by 2.1-fold in comparison with rFVIIa in postdelivery samples. The smaller difference in PT is attributable to the TF in the assay, which serves as a contributor for localizing CT-001 and rFVIIa onto a negatively charged surface for the coagulation reaction. As a result, the amino acid substitution engineered into CT-001 was a less dominant contributor for enhanced binding to a negatively charged surface, a characteristic of these amino acid substitutions that has been reported previously.27,34 However, it should be noted that the therapeutic mechanism of a FVIIa-based molecule to reduce bleeding is independent of TF.28,35 In TGA assays, a CT-001 concentration as low as 3.16 to 10 nM was effective in shortening the lag time and time-to-peak parameters. Higher concentrations of CT-001 and rFVIIa (>100 nM) were needed to induce a mild increase in peak thrombin and ETP. This suggests that CT-001 could increase the rate of thrombin generation to provide hemostatic effect, and, if needed, also increase the total amount of thrombin generation (ETP) by using a higher dose in a hemorrhagic situation. VHA activated by INTEM or NATEM reagent was also evaluated as point-of-care assay for the effects of CT-001 and rFVIIa on postdelivery samples. With INTEM reagent (ellagic acid and calcium chloride), CT-001 and rFVIIa-treated samples had short CTs and a very shallow dose-response curve. This was likely due to the potent coagulation activation by the INTEM reagent. With the NATEM reagent (calcium chloride) reagent, which is less potent in the activation of the coagulation, CT-001 and rFVIIa induced dose-dependent shortening of the average CT measurements. However, the dynamic range of NATEM assay did not have a wide enough dynamic range for some of the patients to demonstrate the procoagulant effect of CT-001 and rFVIIa. Further optimization of the NATEM assay would be needed to enable this point-of-care VHA for monitoring FVIIa treatment. In contrast, standard APTT, PT, and TGA showed wide dynamic assay response to CT-001 and rFVIIa. However, it should be noted that from the experience in hemophilia with inhibitors, thus far there is no FVIIa laboratory assay to directly predict bleeding outcome.36 Therefore, the extent of these assays in predicting the efficacy of CT-001 in PPH remains to be seen in the clinic. A dose ranging from 60 to 90 μg/kg of rFVIIa is being used for PPH.24,29 At this dose, a plasma concentration of 12 to 17 nM is expected after administration, assuming a 70-kg human with 3 L plasma, and a 46% rFVIIa recovery. Because CT-001 exhibited 1.7- to 5.8- (average of 3.8) times higher potency over rFVIIa in the various assays tested, 3.2 to 4.5 nM CT-001 could be its potential concentration for efficacy in human.
The small sample size is a limitation of this study. This pilot study enrolled 28 patients, with 24 patients providing both predelivery and postdelivery blood samples. Moreover, because patients at higher risk of PPH scheduled for cesarean delivery were preferentially enrolled, there was only 1 patient who proceeded into labor in this study. Cesarean delivery could lead to the modulation of clotting activity in a manner that is different from vaginal delivery. Further investigations comparing the 2 delivery routes are warranted. Additionally, only a portion of patients experienced blood loss that met the criteria for severe PPH. To further understand the potency of CT-001 in the setting of severe PPH, a larger study comprising patients with an array of risk factors, including different modes of delivery would be valuable.
The maintenance of peak thrombin generation and upregulation in coagulation activity in the presence of reduced coagulation protein levels through a compensatory mechanism are essential for postpartum hemostasis. The observation of CT-001 being able to enhance the hemostatic activity in postdelivery samples, even in the setting of ongoing coagulation factor consumption, suggests that CT-001 can enhance this unstudied compensatory pathway. Currently, severe PPH nonresponsive to firstline procedures and uterotonics is treated with invasive second-line interventions to control blood loss. Recombinant FVIIa has demonstrated efficacy and was recently approved by the European Medicines Agency but its use has been limited because of thromboembolic risks. The enhanced procoagulant activity of CT-001 observed in this study, together with the previously reported rapid clearance and low thrombogenicity in preclinical studies,27,28 suggest that CT-001 has the potential to be an efficacious therapy for severe PPH and a therapy with potentially increased safety over rFVIIa.
Acknowledgments
The authors thank the patients and their families who agreed to take part in the study.
Authorship
Contribution: D.S.S., T.W.H., S.L., T.A., J.G., S.-C.K., and A.H.J. designed the study; D.S.S., C.R.M., T.W.H., S.L., T.A., J.G., S.-C.K., and A.H.J. performed the study and collected and analyzed data; and D.S.S., C.R.M., T.W.H., D.B., S.L., T.A., J.G., S.-C.K., and A.H.J. wrote the manuscript.
Conflict-of-interest disclosure: D.S.S., C.R.M., T.W.H., and D.B. are employees of and have stock ownerships and stock options in Coagulant Therapeutics. S.L., T.A., J.G., S.-C.K., and A.H.J. received research funding from Coagulant Therapeutics. A.H.J. has received consulting fees from Cerus. T.W.H. is listed as a coinventor on patents filed by Coagulant Therapeutics, related to this study.
Correspondence: Derek S. Sim, Coagulant Therapeutics Corporation, 2630 Bancroft Way, Berkeley, CA 94720; email: dsim@coagulanttherapeutics.com.
References
Author notes
Data are available on request from the corresponding author, Derek S. Sim (dsim@coagulanttherapeutics.com).
The full-text version of this article contains a data supplement.