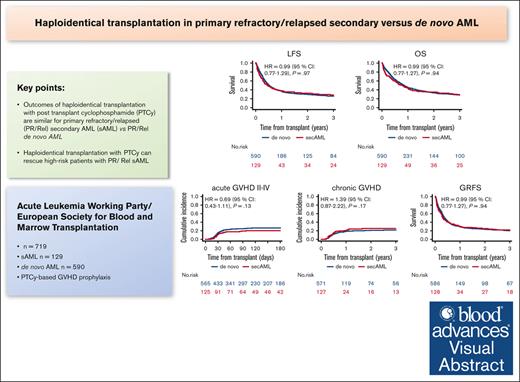
Key Points
Outcomes of haploidentical transplantation with PTCy are similar for primary refractory/relapsed secondary vs de novo AML.
Haplo-HSCT with PTCy can rescue patients with PR/Rel sAML at high risk.
Visual Abstract
We compared the outcomes of haploidentical stem cell transplantation (haplo-HSCT) with posttransplant cyclophosphamide (PTCy) in 719 patients with primary refractory (PR) or first relapse (Rel) secondary acute myeloid leukemia (sAML; n = 129) vs those with de novo AML (n = 590), who received HSCT between 2010 and 2022. A higher percentage of patients with sAML vs de novo AML had PR disease (73.6% vs 58.6%; P = .002). In 81.4% of patients with sAML , the antecedent hematological disorder was myelodysplastic syndrome. Engraftment was 83.5% vs 88.4% in sAML and de novo AML, respectively (P = .13). In multivariate analysis, haplo-HSCT outcomes did not differ significantly between the groups: nonrelapse mortality hazard ratio (HR), 1.38 (95% confidence interval [CI], 0.96-1.98; P = .083), relapse incidence HR, 0.68 (95% CI, 0.4.7.-1.00; P = .051). The HRs for leukemia-free survival, overall survival, and graft-versus-host disease (GVHD)–free, and GVHD and relapse–free survival were 0.99 (95% CI, 0.76-1.28; P = .94), 0.99 (95% CI, 0.77-1.29; P = .97), and 0.99 (95% CI, 0.77-1.27; P = .94), respectively. We conclude that outcomes of haplo-HSCT with PTCy are not different for PR/Rel sAML in comparison with PR/Rel de novo AML, a finding of major clinical importance.
Introduction
Secondary acute myeloid leukemia (sAML) is a subset of AML with notoriously adverse outcomes evolving from an antecedent hematological disorder, mainly myelodysplastic syndromes (MDS) and myeloproliferative neoplasms or as a complication of prior cytotoxic chemotherapy or radiation therapy.1-5 Patients with sAML have inferior outcomes with lower remission rates and overall survival (OS) than those with de novo AML, mainly because of a higher frequency of adverse molecular mutations including secondary type mutations and high-risk cytogenetic abnormalities,6-8 in addition to typically being older and having an antecedent hematological disease.9-12 Allogeneic hematopoietic stem cell transplantation (allo-HSCT) represents a potentially curative therapy in this setting, rescuing up to 40% of patients,13-17 as was already reported in 2010 by the Center for International Blood and Marrow Transplant Research that described 868 patients with therapy-related AML or MDS including those with advanced disease who received HSCT between 1990 and 2004 mainly from matched sibling donors or matched unrelated donors (MUD) and myeloablative conditioning (MAC) with a 5-year disease-free survival and OS of 21% and 22%, respectively.13 On behalf of the Acute Leukemia Working Party (ALWP) of the European Society for Blood and Marrow Transplantation (EBMT), we evaluated transplantation outcomes in ∼5000 patients with sAML who received transplantation between 2000 and 2016, mainly from matched sibling donors and MUD, for which we observed 2-year OS, leukemia-free survival (LFS), and graft-versus-host disease (GVHD)–free, GVHD and relapse–free survival (GRFS) of 44.5%, 38.8%, and 27.2%, respectively.18 Notably, transplantation outcomes in sAML are significantly inferior to those achieved in de novo AML with a lower OS, LFS, and GRFS because of higher nonrelapse mortality (NRM) and relapse incidence (RI).19 Transplantation outcomes are improving, including those for sAML as we have recently demonstrated in a study of patients with sAML comparing 1337 who received transplantation between 2000 to 2010 with 2887 who received transplantation from 2011 to 2020. We demonstrated a significant reduction in the 2-year NRM and a significant improvement in the 2-year GRFS but the 2-year LFS and OS were similar,20 with somewhat better results with MAC vs reduced intensity conditioning.13,21 One of the major advances in the field of transplantation is the development of the non–T-cell-depleted haploidentical stem cell transplantation (haplo-HSCT) with posttransplant cyclophosphamide (PTCy), which has been increasingly used for AML and proven to be highly effective in preventing GVHD and reducing NRM, thus improving transplantation results including for sAML, with a 2-year LFS of 49% and OS of 57% in patients who received transplantation in complete response (CR).22-25 We have recently analyzed outcomes of haplo-HSCT with PTCy in 231 patients with sAML in comparison with 1480 patients with de novo AML, both in first CR (CR1), and observed no significant difference in any transplantation outcome parameter between the sAML vs de novo AML groups,26 which is in contrast to our previous results with HLA-matched allo-HSCT.19 However, results of allo-HSCT may differ in patients with primary refractory (PR) or first relapse (Rel) sAML, a group that is very hard to treat and with substantially inferior transplantation outcomes than patients with leukemia in remission.27,28 Failure to respond to the induction course and relapse are major unfavorable prognostic factors.4,5 PR or Rel AML is associated with a dismal prognosis.4,5,27,28 From a theoretical point of view, it is conceivable that haplo-HSCT will improve results in patients with PR/Rel leukemia, with some reports indicating a stronger graft-versus-leukemia (GVL) effect with haploidentical grafts because of the broad HLA disparity.29,30 We therefore assessed the outcomes of haplo-HSCT in patients with PR/Rel sAML comparing them with those of haplo-HSCT in de novo AML, taking advantage of the ALWP/EBMT registry.
Patients and methods
Study design and data collection
This was a retrospective, multicenter analysis using the data set of the ALWP of the EBMT. The EBMT is a voluntary working group of >600 transplant centers that are required to report all consecutive stem cell transplantations and follow-ups once a year. Since 1 January 2003, all transplantation centers have been required to obtain written informed consent before data registration with the EBMT, as per the Declaration of Helsinki of 1975. Data accuracy is assured by the individual transplant centers and by quality control measures such as regular internal and external audits. In addition, the study protocol was approved by each site and complied with country-specific regulatory requirements. The results of disease assessments at HSCT were also submitted and form the basis of this report. Eligibility criteria for this analysis included adult patients aged ≥18 years with PR/first Rel de novo AML or PR/first Rel sAML who underwent a first HSCT from a non–T-cell-depleted haploidentical donor with PTCy as part of GVHD prophylaxis between 2010 and 2022. Active AML was defined by the failure to achieve CR (bone marrow blasts of >5%) despite induction chemotherapy.27 A haploidentical donor was defined as ≥2 HLA mismatches between donor and recipient. The exclusion criteria were HSCT from other donor types (sibling, unrelated, or cord blood donor), previous history of HSCT, and T-cell–depleted HSCT. Data collected included recipient and donor characteristics including the number of HLA mismatches, age, sex, cytomegalovirus (CMV) serostatus, Karnofsky performance status (KPS) score, and hematopoietic cell transplantation–specific comorbidity index (HCT-CI); disease characteristics including cytogenetics (European LeukemiaNet 2017); and disease status at transplantation, antecedent of malignant disorder, year of transplant, type of conditioning regimen including total body irradiation (TBI), stem cell source, and GVHD prophylaxis regimen including number of immunosuppressive compounds. The conditioning regimen was defined as MAC when containing TBI with a dose of >6 Gray or a total dose of busulfan of >8 mg/kg or >6.4 mg/kg when administered orally or intravenously, respectively. All other regimens were defined as reduced intensity conditioning.31 Grading of acute GVHD (aGVHD) was performed using established criteria.32 Chronic GVHD (cGVHD) was classified as limited or extensive according to published criteria.33 For this study, all necessary data were collected according to the EBMT guidelines, using the EBMT minimum essential data forms. The list of institutions contributing data to this study is provided in the supplemental Appendix.
Statistical analysis
The median, interquartile range (IQR), and range were used for quantitative variables, and frequency and percentage for categorical variables. The study end points were OS, LFS, RI, NRM, engraftment, aGVHD, cGVHD, and GRFS. All end points were measured from the time of transplantation. Engraftment was defined as achieving an absolute neutrophil count of 0.5 × 109/L for 3 consecutive days. OS was defined as time to death from any cause. LFS was defined as survival with no evidence of relapse or progression. NRM was defined as death from any cause without previous relapse or progression. We used modified GRFS criteria. GRFS events were defined as the first event among grade 3/4 aGVHD, extensive cGVHD, relapse, or death from any other cause.34 Patient, disease, and transplant-related characteristics for the 2 cohorts (de novo and sAML) were compared using the Mann-Whitney U test for numerical data, and the χ2 or Fisher exact test for categorical data. Median follow-up was calculated by the reverse Kaplan-Meier method. The probabilities of OS, LFS, and GRFS were calculated using the Kaplan-Meier estimate. The RI and NRM were calculated using cumulative incidence functions in a competing risk setting, with death in remission being treated as a competing event for relapse. Death was considered a competing event for engraftment. To estimate the cumulative incidence of acute or cGVHD, relapse and death were considered as competing events. Univariate analyses were performed using the log-rank test for LFS and OS whereas the Gray test was used for cumulative incidence. Multivariate analyses (MVA) were performed using the Cox proportional-hazards regression model.35 All variables differing significantly between the 2 groups, and potential risk factors were included in the model. To take into account the heterogeneity in the effect of a characteristic or a treatment across centers, we introduce a random effect (or frailty) into the Cox multivariate models.36 We looked at all potential interactions between the core variable and other significant variables. Results were expressed as the hazard ratio (HR) with a 95% confidence interval. All P values were 2-sided with a type 1 error rate fixed at 0.05. Statistical analyses were performed with SPSS 25.0 (SPSS Inc, Chicago, IL) and R 4.0.2 (R Core Team Fifty).37
The scientific boards of the ALWP of the EBMT approved this study.
Results
Patient, transplant, and disease characteristics
A total of 719 patients met the inclusion criteria, 129 with sAML and 590 with de novo AML. Table 1 shows the baseline demographic and clinical characteristics. Median follow-up was 45.59 (IQR, 39.08-57.85) and 43.48 (IQR, 37.53-47.99) months for patients with sAML and de novo AML (P = .2), respectively. Patients with de novo AML were younger, with a median age of 55.4 (range, 18-77.8 years) vs 61.3 (range, 21-78.8 years) years (P < .0001). The median year of transplantation was 2018 (range, 2010-2022) vs 2017 (range, 2010-2022; P = .62) for patients with sAML and de novo AML, respectively; and 65.1% and 57.6% of patients with sAML and de novo AML were male (P = .11), respectively. In patients with sAML, the most frequent (81.4%) antecedent hematological disorder was MDS, followed by another hematological disorder in 10.9% and solid tumor in 8.3% of the patients, respectively. A higher percentage of patients with sAML vs de novo AML had PR disease (73.6% vs 58.6%; P = .002). The distribution of cytogenetic risk was similar between the 2 groups and categorized as intermediate (59.4% vs 58.6%), adverse (37.4% vs 34.4%), and favorable (5.9% vs 4%) for patients with sAML and de novo AML, respectively (P = .75). The KPS score was <90 in 50.8% and 44.1 %, of the patients with sAML and de novo AML, respectively (P = .17). The HCT-CI was higher in the sAML group in comparison with the de novo AML group, with HCT-CI score of ≥3 in 40.3% vs 21.9%, respectively (P < .0001). Both patient and donor CMV seropositivity was similar between the 2 groups, with 79.8% and 75.4% (P = .29), and 53.1% and 59.3% (P = .2) in sAML and de novo AML, respectively. Female donor-to-male patient combination was used in 20.9% of transplants in both sAML and de novo AML. Fewer patients with sAML received MAC than patients with de novo AML, 39.8% vs 47.8%, respectively, but this was not statistically significant (P = .10). Graft source was mainly peripheral blood stem cells in both sAML (69.8%) and de novo (66.8%) groups (P = .51). The most frequent conditioning regimen for both groups was thiotepa/busulfan/fludarabine at 38.8% and 42%, followed by fludarabine/low dose TBI in 17.1% and 12.9%, and busulfan/fludarabine in 14.7% and 16%, of patients with sAML and de novo AML, respectively (supplemental Table 1). For GVHD prophylaxis, PTCy was combined with cyclosporine A and mycophenolate mofetil in 41.4% and 52.9% of the sAML and de novo AML patients, respectively, whereas in 41.1% and 33.6%, respectively, it was combined with mycophenolate mofetil and tacrolimus (supplemental Table 2).
Transplantation outcome
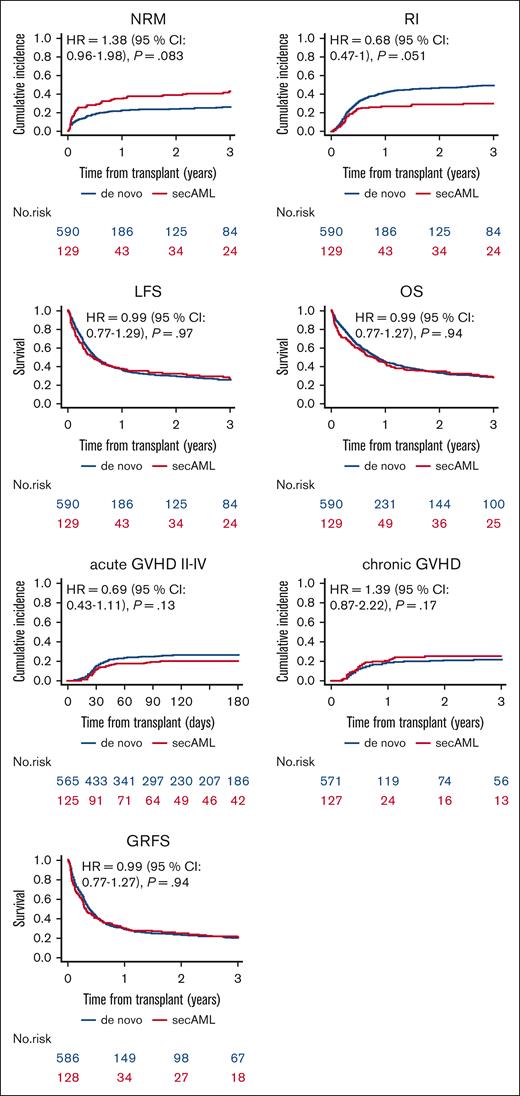
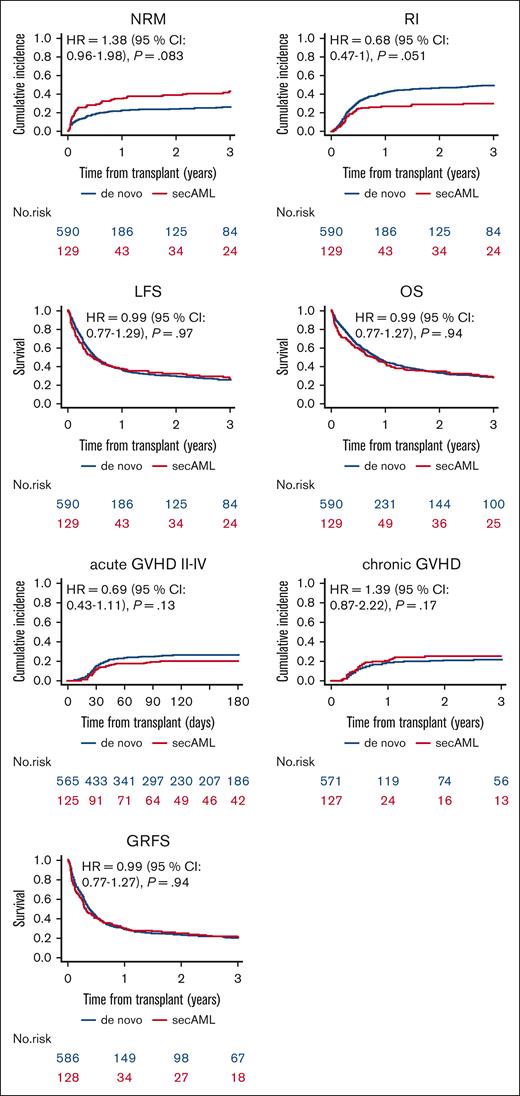
Engraftment and GVHD incidence did not differ between the sAML vs de novo AML groups, as depicted in Table 2. Neutrophil recovery (absolute neutrophil count > 0.5 × 109/L) was achieved in 83.5% and 88.4% of the patients with sAML and de novo, respectively (P = .13). On univariate analysis, on day +180, the incidence of aGVHD grades 2 to 4 and 3/4 was 20% (13.5%-27.4%) vs 26.9% (23.3%-30.6%; P = .12) and 8.9% (4.7%-14.7%) vs 10.4% (8%-13.1%), respectively (P = .61). Two-year incidence of total and extensive cGVHD was 25.3% (17.7-33.5) vs 20.7% (17.3-24.3; P = .27) and 12.5% (7.2-19.4) vs 10.3% (7.9-13.1), respectively (P = .46; Table 3, Figure 1). The outcomes of LFS, OS, and GRFS did not differ between the sAML and de novo AML groups. Two-year NRM and RI were 38.7% (30-47.3) vs 23.8% (20.3-27.4; P = .001) and 28.8% (20.9-37.1) vs 46.3 % (42-50.4; P = .001) in de novo vs sAML, respectively (Table 3, Figure 1). These differences were not confirmed on MVA.
Outcomes of haploidentical transplantation with PTCy in first relapse/PR sAML vs first relapse/PR de novo AML.
Outcomes of haploidentical transplantation with PTCy in first relapse/PR sAML vs first relapse/PR de novo AML.
Multivariate analysis
In the MVA (Table 4), we did not find any statistical difference in transplantation outcomes between the sAML and de novo AML groups. The HRs were 1.38 (0.96-1.98, P = .083) for NRM, 0.68 (0.47-1, P = .051) for RI, 0.99 (0.76-1.28, P = .94) for LFS, 0.99 (0.77-1.29, P = .97) for OS, and 0.99 (0.77-1.27, P = .94) for GRFS (Table 4). Similarly, the risks of aGVHD grade 2 to 4 with a HR = 0.69 (0.43-1.11, P = .13), aGVHD grade 3/4 with a HR = 0.93 (0.47-1.85, P = .84), cGVHD all grades with a HR = 1.39 (0.87-2.22, P = .17), and extensive cGVHD with a HR = 1.13 (0.6-2.15, P = 0. 7) did not differ between the 2 groups (Table 4). Significant prognostic factors were adverse cytogenetics risk associated with higher risk of RI and lower LFS, OS, and GRFS; older age associated with higher NRM and inferior OS; and KPS of ≥90 was a prognostic factor for lower NRM and RI and higher LFS, OS, and GRFS. A peripheral blood graft was associated with a higher risk of aGVHD grade 2 to 4 and 3/4, NRM, and a lower GRFS; and patient CMV seropositivity was associated with a lower OS (Table 4). No difference was observed in any transplantation outcome between patients with PR vs those with Rel (Table 4).
Cause of death
A total of 484 patients died during the study period, comprising 91 with sAML and 393 with de novo AML (Table 5). The original disease was the main cause of death, accounting for 40.9% and 59.3% of the deaths, respectively. The second cause of death was infection at 26.1% and 19.6%, followed by GVHD with 9.1% and 9.4% of deaths, respectively (Table 5). Multiorgan failure accounted for 5.7% and 1.8%, and central nervous system toxicity for 4.5% and 0% of deaths, respectively. Second malignancies accounted for 2.3% and 0.8%, and graft failure/rejection for 3.4% and 0.8% of the deaths, respectively. Other causes of death were infrequent and included veno-occlusive disease of the liver, cardiac toxicity, hemorrhage, and interstitial pneumonitis, each accounting for <1.5% of total deaths with no difference between the patient groups (Table 5).
Discussion
In this study, we have demonstrated similar transplantation outcomes for patients with PR/Rel sAML in comparison with those with de novo AML after non–T-cell-depleted haplo-HSCT with PTCy. Notably, approximately a quarter of this very high-risk group of patients with sAML, with 73.6% being PR, were relapse-free and GVHD-free at 2 years. These results are similar to those published by Brissot et al who compared 199 haplo-HSCT with MUD and MMUD in patients with AML with active disease (PR/Rel) with a 2-year OS of 29.3%, a LFS of 28%, and GRFS of 16.2%.27 Similarly, in a previous study, we assessed transplantation outcomes in 852 patients with AML with active disease by comparing 2 MAC regimens, observing an OS of 31.2% to 33.4% and LFS of 25% to 28.4% at 2 years.38 Comparable data on patients with AML with active disease have been previously published from the Memorial Sloan Kettering Cancer Center and others, in the non–haplo-HSCT setting.39-41 It is with no surprise that the outcome of haplo-HSCT in PR/refractory sAML is worse than that achieved in patients with sAML in remission. In a previous study, we analyzed transplantation outcomes in 154 sAML (45% in CR, 55% with active disease) patients undergoing non-T-depleted haplo-HSCT between 2006 to 2016, and observed a 2-year LFS, OS, and GRFS of 37.1%, 43.3%, and 42.1%, respectively.42 Active disease at the time of transplantation was associated with inferior outcomes, with a 2-year OS of 35.3% compared with 53.2% in patients in CR (P = .02). Active disease vs CR at the time of transplantation was also an unfavorable prognostic factor for LFS with (30.1% vs 45.7%, P = .01) and GRFS (21.5% vs 38.4%, P = .03).42 In a subsequent study that included 246 haplo-HSCT (50% with active disease, and 50% in CR), 2-year LFS, OS, and GRFS were 32%, 41%, and 23%, respectively.43 Again, there was a correlation between disease status at transplantation and outcome. In the MVA, patients who received transplantation in CR had significantly better OS, LFS, and GRFS than those who received transplantation with active disease, with HRs of 1.99, P < .001; 2.17, P < .001, and 1.97, P < .001, respectively. Being with refractory or relapsed leukemia at the time of transplantation may also explain the somewhat lower neutrophil recovery of 83.5% to 88.4% that we observed, which is somewhat similar to previous reports in this setting.28,41
However, none of these studies have focused on comparing outcomes in sAML vs those in de novo AML. Patients with sAML treated with conventional therapy are known to have inferior outcomes, with lower remission rates and OS compared with patients with de novo AML.1,2,9-11 One of the initial questions was therefore whether the same would also be true for patients undergoing transplantation especially because, besides the high-risk disease biology (which may lead to higher posttransplant RI), patients with sAML are typically older, with comorbidities, leading to reduced tolerability of chemotherapy with increased toxicity and side effects,44,45 factors that may result in a higher NRM, both of which will translate into inferior outcomes of allo-HSCT in sAML.21,46 Addressing this question, Schmaelter et al compared transplantation outcomes in 11 439 patients with de novo AML and 1325 with sAML (8600 of whom were in CR1) who received transplantation mostly from sibling and unrelated donors. They observed a higher RI and NRM in patients with sAML vs those with de novo AML, which translated to significantly inferior LFS, OS, and GRFS in patients with sAML with HRs of 1.33, 1.32, and 1.2, respectively.19 We subsequently compared outcomes of haplo-HSCT with PTCy in 231 patients with sAML vs 1480 patients with de novo AML, both in CR1, and observed no significant difference in any transplantation outcome parameter between the 2 groups,26 results that are in contrast to the results of Schmaelter et al in a similar cohort of patients with AML undergoing allo-HSCT from HLA-matched rather than haploidentical donors.19 These, to some degree unexpected, results may be because of a reduction in transplant-related mortality, which is known to be high in sAML transplants13,16,17,19,21,46 because the haplo-HSCT PTCy platform was previously demonstrated to lead to a remarkable reduction in transplant-related mortality and GVHD incidence.22-24,47 As for the 2-year incidence of extensive cGVHD of 20% to 25% that we observed, which may be somewhat higher than previously reported in the haplo-HSCT PTCy setting,22-24 it may be because of early withdrawal of immune suppression used to prevent relapse in this very high-risk population, however being a registry-based study, we do not have this information. Of major importance, particularly for transplantation in patients with active leukemia, is the fact that the haploidentical procedure may be associated with an enhanced antileukemic effect. A stronger GVL effect was recently demonstrated in a mouse-leukemic model in which mismatched cytotoxic T lymphocytes possessed higher cytotoxic activity against the leukemia than their matched counterparts.48 Several clinical studies from China showed faster clearance of posttransplant measurable residual disease (MRD), reduced posttransplant disease progression, and relapse, and better results in patients with high-risk leukemia with positive MRD before transplantation, with haploidentical compared with sibling transplantation.29,30,49 Furthermore, PTCy may provide a direct immune-mediated, specific antileukemic effect, distinct from GVHD, that is probably mediated by the release of cytokines or other molecules to which leukemic cells may be more sensitive than normal cells.50 Notably, using modern immune profiling and machine learning techniques, unique immune signatures and T-cell subset reconstitutions were recently demonstrated with PTCy, which may allow a potent GVL effect while reducing GVHD.51 Indeed, PTCy was shown to impair the proliferation and cytokine production of alloreactive T cells but did not completely eradicate them and thus reduce the progression of severe forms of GVHD while maintaining the GVL effect.52 However, from a clinical point of view, the possible stronger GVL effect associated with haplo-HSCT may not be translated to a reduced relapse rate due to the HLA-loss phenomenon, which is 1 of the major mechanisms of relapse after haplo-HSCT.53,54
Altogether, the reduced toxicity and potentially stronger antileukemic effect associated with haplo-HSCT may explain the lack of difference we observed with the haplo-HSCTs in patients with PR/Rel sAML vs those with PR/Rel de novo AML. The other factors observed to be associated with haplo-HSCT outcomes included cytogenetic risk, age, KPS, CMV seropositivity, and peripheral blood grafts, and are in agreement with previous publications of allogeneic transplantations including haplo-HSCTs in sAML.16,18,19,26 This study, being a retrospective and registry-based transplantation study, has several limitations including the risk of selection bias and the possibility of unavailable data that could not have been considered, such as frontline therapies as well as the number of bone marrow and peripheral blood blasts, mutation profile, molecular, and MRD data.
In conclusion, in this real-life registry-based retrospective analysis of haplo-HSCT for PR/Rel sAML in comparison with haplo-HSCT in PR/Rel de novo AML, we observed similar transplantation outcomes with haplo-HSCT, with approximately a quarter of the very high-risk group of sAML patients, 73.6% being PR, reaching relapse-free and GVHD-free status at 2 years. Hopefully, with the recent advances in our understanding of the biology of sAML as well as the approval of novel agents including vyxeos (CPX-351) and venetoclax,55,56 it may be possible to further improve PR/Rel sAML outcomes.
Acknowledgments
The authors thank all EBMT centers and national registries for contributing patients to this study (see supplemental Appendix). The authors also thank the data managers for their excellent work.
Authorship
Contribution: A.N. wrote the manuscript, designed the study, and interpreted the data; M.L. and M.M. designed the study, performed the statistical analyses, interpreted the data, and edited the manuscript; J.T., A.M.R., D.K., J.V., D.B., P.C., R.F., J.W., E.F., G.V.G., and F.C. reviewed the manuscript and provided clinical data; A.N., M.L., F.C., and M.M had full access to all study data; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Arnon Nagler, Division of Hematology, Chaim Sheba Medical Center, Bone Marrow Transplantation, Tel Hashomer, 52621, Israel; email: arnon.nagler@sheba.health.gov.il.
References
Author notes
Presented, in part, as an oral presentation at the American Society of Hematology, San Diego, CA, 11 December 2023 (abstract 1049).
Data are available on request from authors, Arnon Nagler (arnon.nagler@sheba.health.gov.il), Myriam Labopin, Fabio Ciceri, and Mohamad Mohty.
The full-text version of this article contains a data supplement.