Key Points
There is a need for nuanced consensus guidance on how to initiate and modify treatment for SAA based on individual patient characteristics.
Expert panel provided recommendations for >600 varying scenarios of patients with SAA for initial and subsequent management of SAA.
Visual Abstract
Severe aplastic anemia (SAA) is a rare hematologic condition for which there is no clear management algorithm. A panel of 11 experts on adult and pediatric aplastic anemia was assembled and, using the RAND/University of California, Los Angeles modified Delphi panel method, evaluated >600 varying patient care scenarios to develop clinical recommendations for the initial and subsequent management of patients of all ages with SAA. Here, we present the panel’s recommendations to rule out inherited bone marrow failure syndromes, on supportive care before and during first-line therapy, and on first-line (initial management) and second-line (subsequent management) therapy of acquired SAA, focusing on when transplant vs medical therapy is most appropriate. These recommendations represent the consensus of 11 experts informed by published literature and experience. They are intended only as general guidance for experienced clinicians who treat patients with SAA and are in no way intended to supersede individual physician and patient decision making. Current and future research should validate this consensus using clinical data. Once validated, we hope these expert panel recommendations will improve outcomes for patients with SAA.
Introduction
Acquired aplastic anemia (AA) is an immune-mediated bone marrow (BM) failure disorder, characterized by cytopenias and a hypocellular BM.1-3 Although the precise etiology of autoimmunity in AA remains poorly understood, the last few decades have brought much progress to understanding dysregulated immune responses and autoimmune attack in AA.4-17 The estimated annual incidence of AA is ∼2 cases per million globally.1,18-22 Although toxic exposures (eg, benzene), medications (eg, chloramphenicol and antiepileptics), and infections have been associated with some cases of acquired AA, in most cases an inciting event cannot be identified.1 AA is most often diagnosed in middle to late childhood or late in life.1
Acquired AA remains a diagnosis of exclusion, as there are other acquired or inherited bone marrow failure (IBMF) syndromes that may present similarly; a comprehensive diagnostic evaluation, including clinical and family history, physical examination, and laboratory testing with genetic diagnostics can help to establish the diagnosis.23 Historically, AA has been classified using the modified Camitta criteria into nonsevere/moderate AA, severe AA (SAA), and very severe AA. SAA is defined by marrow cellularity <25% (or 25%-50% with <30% residual hematopoietic cells), plus at least 2 of the following peripheral blood findings: neutrophils of <0.5 × 109/L, platelets of <20 × 109/L, and reticulocytes of <60 × 109/L.24,25 Very SAA fulfills the same criteria as SAA, except neutrophils are <0.2 × 109/L.26,27 Without effective treatment, patients with SAA are at high risk of death from infection or hemorrhage.
Patients with SAA are typically treated with either allogeneic hematopoietic stem cell transplantation (allo-HSCT) or immunosuppressive therapy (IST) alongside a thrombopoietin receptor agonist (TPO-RA) eltrombopag. Allo-HSCT is generally recommended as first-line treatment for younger patients with a matched related donor (MRD), whereas IST is the recommended first line for older adults and those without a fully matched donor.28 However, improving treatment outcomes with the use of unrelated and alternative donors suggest that earlier incorporation of allo-HSCT into the SAA treatment algorithm may be feasible.29-40
The recommended front-line IST consists of horse antithymocyte globulin (ATG) and cyclosporine (CsA), which, when used together with eltrombopag, results in an overall initial response rate of ∼80%.41-44 Among those who do not respond to front-line IST, allo-HSCT or a second course of IST with or without TPO-RAs can be considered. Eltrombopag was first approved in 2014 for the treatment of relapsed/refractory SAA and subsequently approved for use along with IST for treatment-naïve SAA in 2018.42-46 Other TPO-RAs are also in development, and emerging data demonstrate that these also have activity in SAA.47-49
Although previous SAA treatment recommendations exist,50,51 nuanced guidance on how to initiate and modify treatment for SAA based on individual patient characteristics (eg, medical fitness, age, and type of available donor) or disease characteristics (eg, response to prior treatment) is limited. Based on this need, we conducted a RAND/University of California, Los Angeles (UCLA) modified Delphi panel to develop clinical consensus on how to treat patients with SAA.52-57 Although the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) criteria are the gold standard criteria used to develop evidence-based guidelines,58 the RAND/UCLA modified Delphi panel method59 is a validated process for obtaining consensus in clinical management in areas in which optimal management is unclear because of limited, emerging, or changing clinical data, through the use of iterative structured surveys and group discussion of clinical experts.54,56,57 Using this process, we have assembled an 11-expert panel and analyzed 697 individual patient care scenarios across a spectrum of patient ages, physical fitness, initial vs subsequent therapy, and available donor type. Here, we present recommendations as a reference for use by the hematology community along with guidance summarizing consensus and various areas in which expert consensus could not be reached.
Materials and methods
The RAND/UCLA modified Delphi panel method59 is a formal group consensus process that systematically and quantitatively combines expert opinion and evidence by asking physicians to rate, discuss, and then re-rate items. In brief, the steps include selecting a panel of physicians, summarizing the current evidence on the panel topic, and developing a survey collaboratively with panelists. Panelists then independently completed a first-round (premeeting) survey before attending a moderated meeting, during which areas of disagreement on the survey are discussed. Only aggregate results are presented. Panelists then repeat the survey, and these second-round ratings are used to develop clinical consensus recommendations. This process is consistent with elicitation methods recently recommended as best practice for healthcare decision making and for conducting health technology assessments.52
For this report, a diverse 11-member physician panel, comprising 8 adult and 3 pediatric hematologists, was convened. The panel was double blinded; while work was ongoing, the sponsor did not know the identity of the physicians (except for the panel chair), and the physicians were not informed of the sponsor until after consensus recommendations were developed. The sponsor did not provide input on the methodology or results of the panel. The study did not involve human patients as defined by 45 Code of Federal Regulations part 46 and was therefore not subject to institutional review board approval.
Expert panelists were provided with a summary of current evidence on the therapeutic management of patients with SAA before the panel meeting: the etiological differences between inherited and acquired AA, diagnostic evaluation of patients with suspected SAA, and treatment trajectory of patients upon diagnosis. In the evidence review, we included 5 reviews,2,50,51,60,61 1 prospective cohort study,62 7 prospective randomized control trials,24,27,44,63-67 and 4 clinical trials served as significant resources for the literature review.42,45,46,68 Consensus recommendations in this manuscript were also informed by additional studies.41-44,69-74
In collaboration with the expert panelists, a survey (rating form) comprising 697 patient scenarios that varied by factors identified as affecting therapy choice (eg, age, medical fitness defined by Eastern Cooperative Oncology Group [ECOG] criteria, and donor availability) was developed. A copy of the rating form is in the supplemental Appendix. For each scenario, experts rated the appropriateness of interventions for SAA on a 1-to-9 scale (9 being highly appropriate, and 1 being highly inappropriate), including treatments in first- and later-line settings. Panelists also rated the appropriateness of diagnostic testing to rule in SAA or rule out IBMF syndromes as well as rated various supportive care measures (including transfusion support and prophylactic antibiotics) through a patient’s therapeutic lifetime. Physicians completed the ratings and a brief survey independently before a virtual group meeting (Round 1).
At the professionally moderated meeting, physicians reviewed Round 1 results and discussed areas of disagreement. After the meeting, physicians repeated their ratings (Round 2). A median rating was calculated for each item and categorized into 3 groups to represent inappropriate, uncertain, and appropriate ratings (1-3, 4-6, and 7-9). Agreement was defined as no more than 2 ratings outside the category with the group median. Using these classifications, and following several rounds of written review, we developed the following consensus recommendations for the management of SAA, which were approved by all panelists.
Results
In total, we evaluated 697 patient scenarios varied by age, donor availability, performance status, previous therapy, and patient response (supplemental Table 1; Figures 1-4). All Round 2 ratings are provided in the supplemental Appendix. After a structured discussion, 11 panelists agreed on 87% of their Round 2 ratings (Table 1), a significant increase from the proportion of agreement in Round 1 (43%). Below, we summarize the expert recommendations based on areas of consensus. All recommendations below are intended only as general guidance for experienced clinicians who treat patients with SAA. They are in no way intended to supersede individual physician and patient decision making.
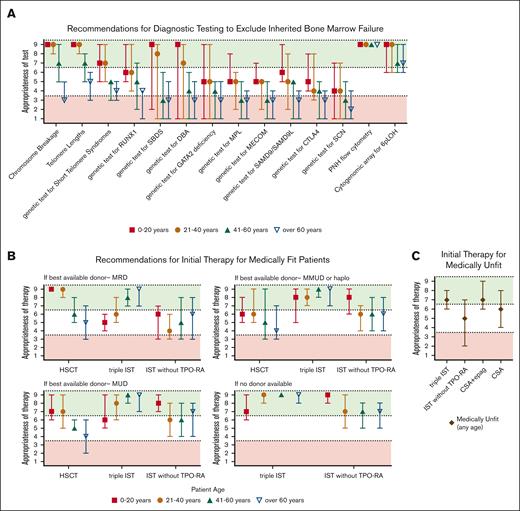
Summary of Delphi panel Round 2 expert consensus ratings for diagnostic testing and initial therapy for SAA. (A) Summary plot of recommended testing to exclude inherited BM failure and “rule in” acquired AA in the evaluation of patients presenting with SAA of different ages. (B) Summary plot of recommended initial therapy in medically fit patients whose best available potential allogeneic donor options are MRD, MUD, mismatched unrelated or haploidentical donor (MMUD or haplo), or no donor available (or unknown). (C) Summary plot of recommended initial treatment for medically unfit patients. In all cases, data represent median score ± 95% confidence interval (CI). Scores in the range of 7 to 9 (green color) indicate treatments that would be highly appropriate. Scores in the range of 1 to 3 (red color) indicate treatments that would be less appropriate. Intermediate scores in the range of 4 to 6 (white color) indicate treatments that may be appropriate under selected circumstances.
Summary of Delphi panel Round 2 expert consensus ratings for diagnostic testing and initial therapy for SAA. (A) Summary plot of recommended testing to exclude inherited BM failure and “rule in” acquired AA in the evaluation of patients presenting with SAA of different ages. (B) Summary plot of recommended initial therapy in medically fit patients whose best available potential allogeneic donor options are MRD, MUD, mismatched unrelated or haploidentical donor (MMUD or haplo), or no donor available (or unknown). (C) Summary plot of recommended initial treatment for medically unfit patients. In all cases, data represent median score ± 95% confidence interval (CI). Scores in the range of 7 to 9 (green color) indicate treatments that would be highly appropriate. Scores in the range of 1 to 3 (red color) indicate treatments that would be less appropriate. Intermediate scores in the range of 4 to 6 (white color) indicate treatments that may be appropriate under selected circumstances.
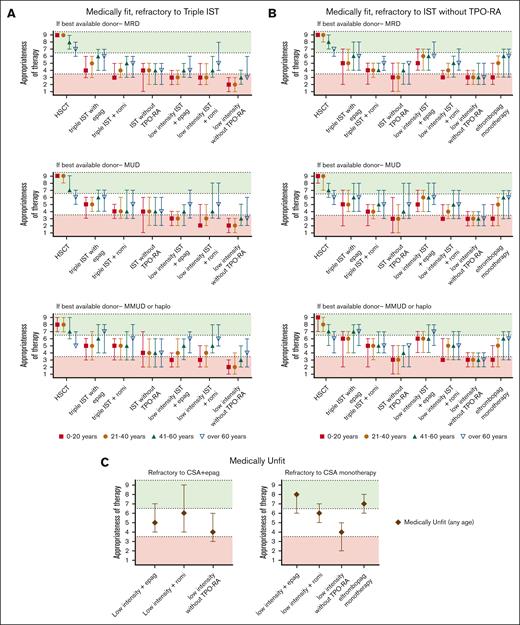
Summary of Delphi panel Round 2 expert consensus ratings for subsequent therapy in patients refractory to initial treatment course. (A) Summary plot of recommended subsequent therapy in medically fit patients who are refractory to “triple IST” (horse ATG, CsA, or eltrombopag) based on the best available allogeneic donor option. (B) Summary plot of recommended subsequent therapy in medically fit patients who are refractory to standard IST administered without TPO-RA stratified based on the best available allogeneic donor option. (C) Summary plot of recommended subsequent therapy in medically unfit patients refractory to the initial treatment of CsA eltrombopag (CsA + eltrombopag), or to CsA monotherapy. In all cases, data represent median score ± 95% CI. Scores in the range of 7 to 9 (green color) indicate treatments that would be highly appropriate. Scores in the range of 1 to 3 (red color) indicate testing that would be less appropriate. Intermediate scores in the range of 4 to 6 (white color) may be appropriate under selected circumstances.
Summary of Delphi panel Round 2 expert consensus ratings for subsequent therapy in patients refractory to initial treatment course. (A) Summary plot of recommended subsequent therapy in medically fit patients who are refractory to “triple IST” (horse ATG, CsA, or eltrombopag) based on the best available allogeneic donor option. (B) Summary plot of recommended subsequent therapy in medically fit patients who are refractory to standard IST administered without TPO-RA stratified based on the best available allogeneic donor option. (C) Summary plot of recommended subsequent therapy in medically unfit patients refractory to the initial treatment of CsA eltrombopag (CsA + eltrombopag), or to CsA monotherapy. In all cases, data represent median score ± 95% CI. Scores in the range of 7 to 9 (green color) indicate treatments that would be highly appropriate. Scores in the range of 1 to 3 (red color) indicate testing that would be less appropriate. Intermediate scores in the range of 4 to 6 (white color) may be appropriate under selected circumstances.
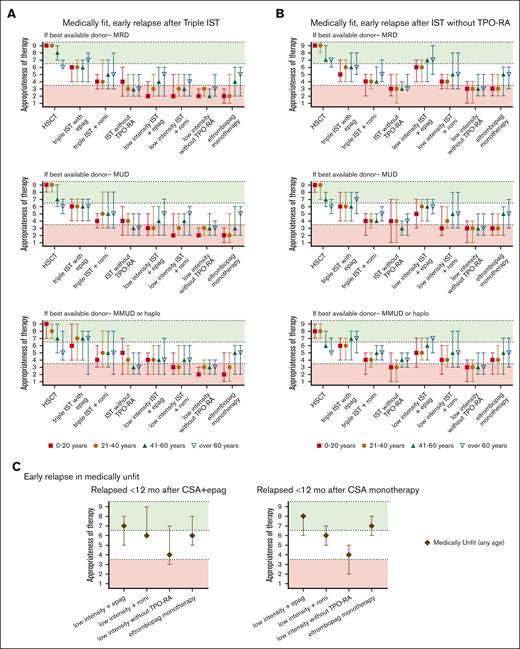
Summary of Delphi panel Round 2 expert consensus ratings for subsequent therapy in patients who relapse within 12 months of initial therapy and again develop severe cytopenias meeting the criteria for SAA. (A) Summary plot of recommended subsequent therapy in medically fit patients who relapse within 12 months of “triple IST” (horse ATG, CsA, or eltrombopag) based on the best available allogeneic donor option. (B) Summary plot of recommended subsequent therapy in medically fit patients who relapse within 12 months of standard IST administered without TPO-RA stratified based on the best available allogeneic donor option. (C) Summary plot of recommended subsequent therapy in medically unfit patients who relapse within 12 months of initial treatment of CsA eltrombopag (CsA + eltrombopag), or CsA monotherapy. In all cases, data represent median score ± 95% CI. Scores in the range of 7 to 9 (green color) indicate treatments that would be highly appropriate. Scores in the range of 1 to 3 (red color) indicate testing that would be less appropriate. Intermediate scores in the range of 4 to 6 (white color) may be appropriate under selected circumstances.
Summary of Delphi panel Round 2 expert consensus ratings for subsequent therapy in patients who relapse within 12 months of initial therapy and again develop severe cytopenias meeting the criteria for SAA. (A) Summary plot of recommended subsequent therapy in medically fit patients who relapse within 12 months of “triple IST” (horse ATG, CsA, or eltrombopag) based on the best available allogeneic donor option. (B) Summary plot of recommended subsequent therapy in medically fit patients who relapse within 12 months of standard IST administered without TPO-RA stratified based on the best available allogeneic donor option. (C) Summary plot of recommended subsequent therapy in medically unfit patients who relapse within 12 months of initial treatment of CsA eltrombopag (CsA + eltrombopag), or CsA monotherapy. In all cases, data represent median score ± 95% CI. Scores in the range of 7 to 9 (green color) indicate treatments that would be highly appropriate. Scores in the range of 1 to 3 (red color) indicate testing that would be less appropriate. Intermediate scores in the range of 4 to 6 (white color) may be appropriate under selected circumstances.
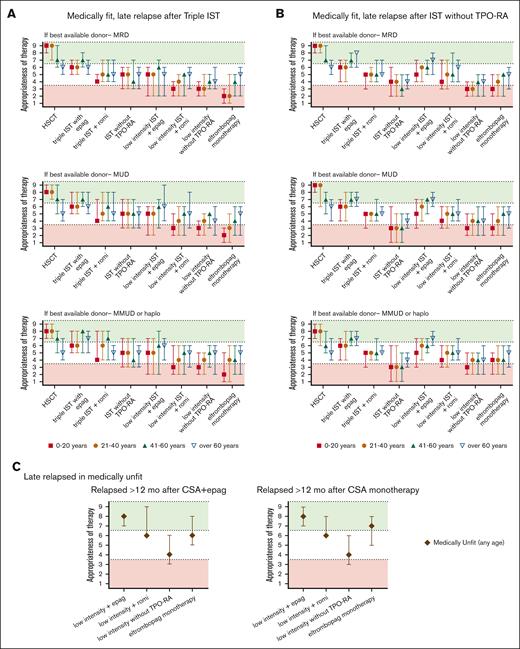
Summary of Delphi panel Round 2 expert consensus ratings for subsequent therapy in patients with late relapses (after 12 months of initial therapy) who again develop severe cytopenias meeting 3 criteria for SAA. (A) Summary plot of recommended subsequent therapy in medically fit patients who relapsed after 12 months of “triple IST” (horse ATG, CsA, or eltrombopag) based on the best available allogeneic donor option. (B) Summary plot of recommended subsequent therapy in medically fit patients who relapsed after 12 months of standard IST administered without TPO-RA stratified based on the best available allogeneic donor option. (C) Summary plot of recommended subsequent therapy in medically unfit patients who relapse after 12 months of initial treatment of CsA eltrombopag (CsA + eltrombopag), or CsA monotherapy. In all cases, data represent median score ± 95% CI. Scores in the range of 7 to 9 (green color) indicate treatments that would be highly appropriate. Scores in the range of 1 to 3 (red color) indicate testing that would be less appropriate. Intermediate scores in the range of 4 to 6 (white color) may be appropriate under selected circumstances.
Summary of Delphi panel Round 2 expert consensus ratings for subsequent therapy in patients with late relapses (after 12 months of initial therapy) who again develop severe cytopenias meeting 3 criteria for SAA. (A) Summary plot of recommended subsequent therapy in medically fit patients who relapsed after 12 months of “triple IST” (horse ATG, CsA, or eltrombopag) based on the best available allogeneic donor option. (B) Summary plot of recommended subsequent therapy in medically fit patients who relapsed after 12 months of standard IST administered without TPO-RA stratified based on the best available allogeneic donor option. (C) Summary plot of recommended subsequent therapy in medically unfit patients who relapse after 12 months of initial treatment of CsA eltrombopag (CsA + eltrombopag), or CsA monotherapy. In all cases, data represent median score ± 95% CI. Scores in the range of 7 to 9 (green color) indicate treatments that would be highly appropriate. Scores in the range of 1 to 3 (red color) indicate testing that would be less appropriate. Intermediate scores in the range of 4 to 6 (white color) may be appropriate under selected circumstances.
Tests to diagnose acquired SAA and rule out IBMF syndromes
Therapy for acquired SAA should not be delayed while awaiting test results to rule out IBMF syndromes unless there are clinical features suggestive of an IBMF. The following should be obtained in all patients: a complete blood count with differential, review of peripheral blood smear, reticulocyte count, paroxysmal nocturnal hemoglobinuria (PNH) assessment by flow cytometry of peripheral blood, and BM biopsy and aspirate with karyotype. Given the poor growth of cytogenetics culture in cases of AA and to exclude hypocellular myelodysplastic syndrome (MDS), fluorescence in situ hybridization (FISH) for MDS-associated cytogenic abnormalities is recommended. Somatic next-generation sequencing (NGS) gene panels for genes frequently mutated in hematologic malignancies have become standard in the evaluation of suspected BM failure.28,75 Although somatic mutations are commonly seen in both AA and in hypocellular MDS, the inclusion of somatic NGS sequencing panels, supported by commonly used practice guidelines, can help with baseline assessment, including identification of MDS-defining variants, and can be informative prognostically and in longitudinal follow-up of clonal evolution.62,76-79 HLA tissue typing should be ordered upfront for patients of all ages who are candidates for HSCT. Comprehensive ancillary studies to exclude transient etiologies of pancytopenia (eg, nutritional deficiencies or viral and other infections) should also be done in all patients.
We recommend obtaining PNH flow cytometry and single-nucleotide polymorphism array genetic testing for chromosome arm 6p loss of heterozygosity clone, because both of these findings are highly predictive of the diagnosis of immune-mediated AA and can be helpful for exclusion of an IBMF.80,81 PNH granulocyte clones of >1% have ∼100% positive predictive value for AA, whereas PNH granulocyte clones of >0.1% were estimated to have 95% specificity and a 91% positive predictive value for the diagnosis of AA.80,82 In contrast, rare PNH-like cells and PNH populations of <0.1% are less specific and have been observed in patients with confirmed IBMF.82
For testing for IBMF (Figure 1A), in patients aged ≤40 years, we recommend chromosome breakage analysis for Fanconi anemia (FA) and telomere length analysis for telomere biology disorders, 2 of the most common groups of IBMFs.83 In patients aged 41- to 60 years, these tests can be considered depending on individual patient circumstances, family history, or clinical presentation. Germ line genetic testing is warranted in patients with a high index of suspicion for an IBMF. These include patients who have a clinical diagnosis of FA based on a positive chromosome breakage tests and patients that have a high clinical suspicion of FA but have a negative chromosome breakage test. An estimated 10% to 25% of patients with FA have somatic reversion mosaicism and have a less severe hematologic presentation; these patients may be missed because of a false-negative chromosome breakage test on peripheral blood lymphocytes and require testing of chromosome breakage in skin fibroblasts.84,85
We recommend IBMF genetic testing in patients with short telomeres suggestive of underlying telomere biology disorders and those with suspected germ line predisposition syndromes. Many known genes associated with IBMF are covered by germ line genetic testing panels offered by academic and commercial genetic laboratories. Depending on the gene content included in the specific NGS panel, testing of additional relevant IBMF genes may have to be ordered separately. Genetic testing should be performed using nonhematopoietic tissue, with gold standard being cultured skin fibroblasts; if hematopoietic tissue was used for initial mutation detection, germ line mutation status should be confirmed using constitutional DNA.
In patients aged >60 years with prior documented normal blood counts and no findings suggestive of IBMF, we do not recommend chromosome breakage analysis for FA or genetic testing for other IBMF syndromes.
Supportive care before and during first-line therapy
Regardless of planned treatment (IST or allo-HSCT), we recommend that a patient with hemoglobin (Hgb) of <7 g/L (or higher Hgb with symptomatic anemia) receive transfusion with leukoreduced, irradiated red blood cells. In patients with <10 ×109/L platelets (or <50 ×109/L if bleeding or if an invasive procedure is planned), we recommend leukoreduced, irradiated platelet transfusion (ideally with single donor platelets to reduce the risk of alloimmunization).
In patients treated with lymphocyte-depleting therapy (eg, ATG, allo-HSCT), we recommend the following prophylactic antimicrobials to be administered until adequate lymphocyte recovery (absolute CD4 T cells of >200 × 103/L) and absolute neutrophil count of >0.50 × 109/L: antibiotic for pneumocystis pneumonia prophylaxis (eg, atovaquone, trimethoprim/sulfamethoxazole, or pentamidine), antifungal prophylaxis (eg, mold active azole), and antiviral prophylaxis for herpes simplex virus and varicella-zoster virus (eg, acyclovir or valacyclovir).2,28,86-89 In line with the Infectious Disease Society of America recommendations for allo-HSCT recipients, we recommend broad-spectrum antibiotic with gram-negative and pseudomonal coverage (eg, fluoroquinolone) until neutrophil recovery in allo-HSCT recipients and patients treated with IST who have prolonged neutropenia (≥7 days).86,90
Initial management
In this section, we present our recommendations for medically fit patients, stratified by age (pediatric [aged ≤20 years] and adult [aged 21-40, 41-60, and >60 years]; Figure 1B).
In medically fit (ECOG score of ≤2) patients91 aged ≤20 years, we recommend the following based on donor availability. In patients with a MRD, we recommend allo-HSCT. For patients without a MRD, we recommend horse ATG + CsA. Given the limited data on the benefits of adding eltrombopag in the pediatric setting, we recommend horse ATG + CsA without eltrombopag in this age group. If available, allo-HSCT from a well-matched unrelated donor (MUD) could also be considered. A randomized clinical trial of IST vs MUD for pediatric patients is currently ongoing.92 Upfront transplant from a haploidentical donor has published success and is currently under investigation in a multicenter Blood and Marrow Transplant Clinical Trials Network trial.31,93 If a haploidentical donor is available, the panel felt that a haploidentical transplant can also be an option with careful clinical consideration.31,93
In patients aged 21 to 40 years, we recommend the following based on donor availability. In patients with a MRD, we recommend allo-HSCT. For other patients, based on the results of the RACE and the National Institute of Health studies in upfront AA, demonstrating the superior hematologic responses of IST with the inclusion of eltrombopag,42,44 we recommend horse ATG + CsA + eltrombopag. If a well-matched MUD is available, allo-HSCT can be considered. Decision making could be driven by various factors, including patients’ health and performance status, weighing relative value for a given patient of the pros/cons of the short-term vs long-term risks of IST vs allo-HSCT, disease features and projected likelihood of response to IST (eg, in patients with favorable vs less favorable prognostic features),62,76,94-98 presence of deep neutropenia at diagnosis, fertility and family planning considerations, and psychosocial issues (eg, motivation/adherence to treatment regimen and availability of caregiver support).99-105 In patients aged >40 years, we recommend horse ATG + CsA + eltrombopag. If these patients have an available MRD or MUD, allo-HSCT can also be considered depending on individual patient circumstances. Upfront transplant from a haploidentical donor is available and, as above, currently under investigation.31,93
Subsequent management
After medical therapy is initiated, eltrombopag should be continued for ∼6 months, provided the patient has achieved a hematologic response, and then tapered or stopped.42,106 Patients should be maintained on full-dose CsA for 6 to 12 months. When adequate hematologic response is achieved, CsA should be tapered slowly over the second year (ie, after the initial 6-12 months full-dose treatment, lower dose CsA maintenance should be maintained for at least 12-18 months, with taper guided by sustained hematologic response). A complete hematologic response is defined as neutrophils of >1 × 109/L (in some trials it is 1.5 ×109/L), platelets of >100 × 109/L, and Hgb of >100 g/L.63 Patients should be monitored for clonal evolution by BM biopsy while on TPO-RA and if there are clinical concerns (eg, a decline in blood counts or lack of response to therapy), with tests including karyotyping, FISH for MDS-associated cytogenetic rearrangements (in the setting of limited or no growth on cytogenetics), and NGS for malignancy-associated mutations.42-44,46,62,76,107,108
Below we present recommendations for treating patients who are refractory (ie, no response at 3-6 months after initial therapy) or who have relapsed (ie, after obtaining a response). Unless the etiology of the patient’s hematologic deterioration is clear with recent BM studies, we recommend repeating BM biopsy for karyotyping, NGS, and FISH to evaluate for MDS-associated clonal evolution. We recognize that relapsing patients will, in most cases, be treated initially by increasing the dose or resuming low-intensity therapy (eg, CsA and/or eltrombopag) to prevent recurrence of severe cytopenias, and that many patients will respond to these initial therapies. The recommendations below are for patients who did not respond to these initial approaches and progressed to meet SAA criteria despite therapeutic interventions.
In medically fit patients91 who received horse ATG + CsA + eltrombopag in a first-line setting, we recommend the following (Figure 2A): in patients aged ≤60 years refractory to initial therapy, we recommend allo-HSCT with the best available donor (eg, MRD, MUD, or haploidentical). In patients aged >60 years refractory to initial therapy, we also recommend allo-HSCT with a MRD, and depending on individual patient circumstances, allo-HSCT with the best-available alternative donor (eg, MUD or haploidentical) may also be considered.
The panel considered clinical scenarios of early relapses (within 12 months of IST; Figure 3) separately from late relapses after 12 months of therapy (Figure 4). This was because early relapses at the time when most patients remain on CsA but discontinue eltrombopag may have a distinct pathogenesis from later immunologic relapses that occur at the time of CsA taper and discontinuation.106,109 However, our recommendations for managing early and late relapses are similar. In patients aged ≤60 years who relapsed after obtaining a response (Figure 3), we recommend either allo-HSCT with the best available donor (MRD, MUD, or haploidentical) or repeating high-intensity medical therapy with eltrombopag or romiplostim. When allo-HSCT is not an option in the refractory or relapsed setting, we recommend high-intensity medical therapy (Table 1; eg, rabbit ATG + CsA, horse ATG + CsA, alemtuzumab, in combination with eltrombopag). Recent data showed that romiplostim also has activity in relapsed/refractory AA.110-113 We also recommend evaluating all patients for available clinical trials.
In medically fit patients who received horse ATG + CsA as first-line therapy, we recommend the following: in patients aged ≤60 years refractory to initial therapy (Figure 2B) or relapsed after obtaining a response, we recommend allo-HSCT with the best available donor (MRD, MUD, or haploidentical). In patients aged >60 years refractory to initial therapy or relapsed after obtaining a response, we also recommend allo-HSCT with an MRD; allo-HSCT with a best available alternative donor (eg, MUD or haploidentical) may be considered in patients depending on individual patient circumstances. For patients who relapsed after obtaining a response (Figure 3B), a second course of high-intensity therapy or resuming CsA with eltrombopag can be attempted to reinduce response; we recommend this option particularly in patients with late immune-type AA relapses. In patients aged >20 years, when allo-HSCT is not a medical option, we recommend high-intensity or low-intensity medical therapy in combination with eltrombopag (Table 2). In patients aged ≤20 years, when allo-HSCT is not a medical option, we did not agree on whether eltrombopag should be provided with second-line medical therapy because of limited data for this option in young patients. We also recommend evaluating all patients for available clinical trials.
In all medically unfit patients91 regardless of age (Figures 2C and 3C), we recommend the following: low-intensity therapy (Table 2) with eltrombopag; single-agent eltrombopag in patients who did not receive eltrombopag in the first-line setting; romiplostim can also be considered depending on patient-specific clinical context. Switching to romiplostim may be particularly advantageous in patients intolerant to eltrombopag, such as those with Gilbert syndrome, difficulty with dietary restrictions, and those with insufficient response on eltrombopag for whom dose escalation with romiplostim can be helpful.111-113
Discussion
In this study, we used a RAND/UCLA modified Delphi panel process to develop expert consensus recommendations for the treatment of SAA. SAA is a relapsing/remitting autoimmune disease that can be managed with differing therapies ranging from allo-HSCT to single-agent low-intensity therapies. Recent advances in transplant and nontransplant therapies and supportive care have improved patient outcomes, but evidence comparing therapeutic modalities and their optimal use over the disease course is lacking. Given the rarity of AA and changing clinical landscape of available therapies, we convened a panel of 11 AA experts to consider various aspects of initial and subsequent management of patients with SAA to develop consensus recommendations. Through the iterative consensus process, we evaluated 697 individual patient care scenarios spanning the spectrum of patient age, medical fitness, best donor availability, initial or subsequent line of therapy, and response to prior therapies, and present our expert assessments of the management of diverse AA clinical scenarios. Our expert panel recommendations presented here summarize areas consensus and areas of active discussion for which expert consensus on optimal management was not reached.
During the Delphi process, treatment options were considered individually within each clinical scenario. Therapies with proven efficacy were rated as appropriate with varying strengths of the recommendation based on whether this was a preferred or a less-preferred option based on a patient’s clinical characteristics and response to prior therapies. For example, in considering initial therapies in younger medically fit patients with an available MRD donor, allo-HSCT was the preferred option, but IST-based regimens were also rated as potentially appropriate under selected circumstances. In doing so, we recognized that, in practice, patients may do well with IST-based regimens, which they may select over allo-HSCT because of personal considerations (eg, fertility, educational, financial, or psychological factors).
Improved allo-HSCT outcomes with unrelated and haploidentical donors are reflected in the panel’s recommendation of allo-HSCT using MRDs or MUDs as the preferred initial therapy in younger, medically fit patients. The panel recommended salvage allo-HSCT using either MRDs, MUDs, or haploidentical donors in younger medically fit patients who were refractory or relapsed to meet SAA criteria after initial therapy. Our panel took place before the availability of the more recent studies reporting encouraging outcomes using haploidentical transplant donors in the upfront setting31,35,36,114-116 and we are not yet able to provide consensus recommendations on the role haploidentical donor allo-HCST in upfront management of AA. Similarly, the panel could not clearly recommend allo-HSCT over IST outright in patients whose best donor was a MUD because there is not yet clear clinical support for this recommendation, but the panel felt that in practice upfront MUD allo-HSCT could be appropriate in younger fit patients with careful clinical consideration. A multicenter randomized study comparing MUD vs IST for pediatric and young adults with SAA is ongoing (ClinicalTrials.gov identifier: NCT05600426).92 Of course, the use of each of these therapies varies by patient characteristics, including age, donor availability, medical fitness, response to prior therapies, and physician input. Detailed recommendations on when different therapeutic options should be initiated or changed based on these characteristics will facilitate better patient care.
The addition of eltrombopag to upfront IST has improved overall response rates to nearly 80%, with faster and more complete hematologic recovery.42,44,106 Our panel recommended the inclusion of eltrombopag in high- and low-intensity IST regimens as the preferred nontransplant treatment option. At the time of our panel, romiplostim had been shown to also have efficacy in SAA, particularly during second- or later-line treatment111-113; and the panel recommended romiplostim to be considered as an alternative, particularly during subsequent therapy and in cases of insufficient response or intolerance of eltrombopag. Since the time of the panel, early data demonstrate encouraging efficacy of other TPO-RAs (eg, avatrombopag and hetrombopag) in AA therapy.47-49 Further research is needed to provide clear guidance on comparative effectiveness and safety of the newer TPO-RA and optimal sequencing in first-line vs subsequent therapy. The evidence on the efficacy of eltrombopag in subsequent management has been less clear in the pediatric population,117 and our panel acknowledges that limitation by not uniformly recommending it in patients aged ≤20 years.
There are gaps in our recommendations. We did not specify the amount of time that patient blood counts may be trending down after loss of hematologic response before initiating high-intensity therapy. Depending on clinical scenario at the time of relapse (eg, whether loss of hematologic response was temporally related to the taper or discontinuation of CsA or discontinuation of eltrombopag) we recommend early therapeutic intervention (eg, resuming eltrombopag or CsA, or intensifying CsA dose), before patients once again meet the criteria for SAA. We did not attempt to develop a comprehensive list of all genetic tests needed to rule out IBMF or germ line predisposition syndromes. Our panel did agree that for patients who do not have overt findings of IBMF, the care of AA should not be delayed while awaiting the results of genetic testing, and recommended functional screening with chromosome breakage and telomere length measurements for all but the oldest patients. Recent publications have presented recommendations for additional genetic testing,23,51,118-120 the specifics of which may depend on institutional availability, patient age, or other presenting characteristics. Additional work is needed to determine optimal genetic testing in the evaluation of suspected idiopathic SAA in adults.
To generate our recommendations, we used the RAND/UCLA modified Delphi panel method, which has been used extensively to develop quality measures and clinical guidance in a variety of areas. This method is distinct from evidence-based recommendations produced using the GRADE system, which rely on the availability of evidence and the classification of such evidence into 4 levels (high, moderate, low, and very low). The quality of evidence helps determine the strength of resulting guidelines.121 Consensus recommendations developed using the Delphi panel method have content, construct, and predictive validity,53 and have been shown to improve health outcomes.54,55 Nevertheless, the method still relies on physician opinion. Although all physicians had significant experience treating patients with SAA and represented diverse backgrounds, practice settings, and geographic regions, 11 people cannot represent the full experience of clinicians who work in this field. Furthermore, our panel included only experts from the United States, so the consensus recommendations may not be generalizable to care in other countries. Although the modified Delphi method does have reasonable reproducibility,56 different groups of experts may have reached different conclusions. We hope that our Delphi consensus ratings for individual scenarios along with the spread of scores within the panel (Figures 1-4) will be useful to the community as an expert discussion of the relative appropriateness of treatment options and areas of disagreement in the field.
These consensus statements were developed with the goal of improving the quality of care delivered to patients with SAA, informed by currently available evidence and expert clinical opinion. In this setting, the guidance is unable to capture the nuance that will vary in individual patients (eg, insurance coverage, cost of therapy, practice setting, and other social determinants of health) and are intended as general consensus recommendations only. In practice, other clinical and nonclinical factors beyond those addressed here may affect optimal SAA management. Still, the hope is to ultimately offer the best available therapy to each patient with SAA.
Acknowledgments
This study was supported by research funding from Novartis to D.V.B., A.E.D., C.D., Z.R.R., S.F., R.H., J.P.M., B.S., S.T., M.W.W., and B.J.P. D.B., M.S.B., S.N.G., and I.Y. are employees of Partnership for Health Analytic Research, which was paid by Novartis to conduct the research described in this manuscript.
Authorship
Contribution: All authors have met International Committee of Medical Journal Editors authorship requirements. D.B., M.S.B., S.N.G., and I.Y. designed and performed research; D.V.B., D.B., M.S.B., S.N.G., I.Y., and B.J.P. analyzed data, wrote the first draft of the manuscript, and revised the manuscript; and A.E.D., C.D., Z.R.R., S.F., R.H., J.P.M., B.S., S.T., and M.W.W. contributed to research, reviewed and critiqued data analysis, and reviewed and critiqued the manuscript.
Conflict-of-interest disclosure: The authors have completed International Committee of Medical Journal Editors Disclosure of Interest forms. D.V.B. reports support for the present manuscript for participation in the Delphi panel outlined in the manuscript by Novartis; reports research grants from the National Institutes of Health and the American Society of Hematology (ASH); reports payment or honoraria for participation in Highlights of ASH from the American Society of Hematology; and reports stock or stock options from Carisma Inc. A.E.D. reports support for the present manuscript for participation in the Delphi panel outlined in the manuscript by Novartis; reports consulting fees from Geron, Regeneron, Sobi, Caribou, Apellis, Bristol Myers Squibb, and Novartis. C.D. reports support for the present manuscript for participation in the Delphi panel outlined in the manuscript by Novartis; reports consulting fees from Novartis for a steering committee; reports payment or honoraria from Alexion and Apellis; and reports participation on a data safety monitoring board or advisory board from Regeneron. Z.R.R. reports support for the present manuscript for participation in the Delphi panel outlined in the manuscript by Novartis; reports honoraria for lecture on Bone Marrow Failure for Sub-specialty Board Review Course (2021) from the American Society of Pediatric Hematology/Oncology; reports payment or honoraria from UpToDate as author of Schwachman Diamond Topic Card; reports payment or honoraria from WebMD (formerly Medscape) as author of Pearson’s Syndrome Topic; reports participation on a data safety monitoring board or advisory board as a panel member for the Federal Data Safety Monitoring Board (DSMB #1) for the National Institutes of Health/the National Heart, Lung, and Blood Institute Bone Marrow Transplant Clinical Trials Network; reports leadership as past chair, with review of AAP hematology-related issues unpaid for the American Academy of Pediatrics Section on Hematology Oncology executive committee; and reports other financial or nonfinancial interests for programmatic review/grant review panel member as the chair from 2023 from the Bone Marrow Failure Research Program of the Congressionally Mandated Research Program. D.B. is an employee of Partnership for Health Analytic Research, which was paid by Novartis to conduct the research described in this manuscript, and by Akcea, Amgen, BioMarin Pharmaceuticals, Bristol Myers Squibb, Celgene, Delfi Diagnostics, Dompe, Eisai, Exact Sciences Corporation, Genentech, Gilead, GRAIL, Greenwich Biosciences, Ionis, Jazz, Nobelpharma, Novartis, Otsuka, Pfizer, Recordati, Regeneron, Sanofi US Services and Takeda Pharmaceuticals USA to conduct research related to the work described in this manuscript and outside of this submitted work. M.S.B. is an employee of Partnership for Health Analytic Research, which was paid by Novartis to conduct the research described in this manuscript, and by Akcea, Amgen, BioMarin Pharmaceuticals, Bristol Myers Squibb, Celgene, Delfi Diagnostics, Dompe, Eisai, Exact Sciences Corporation, Genentech, Gilead, GRAIL, Greenwich Biosciences, Ionis, Jazz, Nobelpharma, Novartis, Otsuka, Pfizer, Recordati, Regeneron, Sanofi US Services, and Takeda Pharmaceuticals USA to conduct research related to the work described in this manuscript and outside of this submitted work. S.F. reports support for the present manuscript for participation in the Delphi panel outlined in the manuscript by Novartis; reports consulting fees from Genentech and Incyte; reports payment or honoraria from Takeda, Bristol Myers Squibb, and Sanofi; reports participation on a data safety monitoring board or advisory board from Genmab and AbbVie; and reports leadership or fiduciary role in other board, society, committee, or advocacy group paid or unpaid from South Carolina Oncology Society. S.N.G. is an employee of Partnership for Health Analytic Research, which was paid by Novartis to conduct the research described in this manuscript, and by Akcea, Amgen, BioMarin Pharmaceuticals, Bristol Myers Squibb, Celgene, Delfi Diagnostics, Dompe, Eisai, Exact Sciences Corporation, Genentech, Gilead, GRAIL, Greenwich Biosciences, Ionis, Jazz, Nobelpharma, Novartis, Otsuka, Pfizer, Recordati, Regeneron, Sanofi US Services, and Takeda Pharmaceuticals USA to conduct research related to the work described in this manuscript and outside of this submitted work. R.H. reports support for the present manuscript for participation in the Delphi panel outlined in the manuscript by Novartis. J.P.M. reports support for the present manuscript for participation in the Delphi panel outlined in the manuscript by Novartis; reports grants or contracts from National Institutes of Health, Leukemia and Lymphoma Society, and US Department of Defense; reports consulting fees for participation on an advisory board from Regeneron and Novartis, and consulting fees for MOS Registry from Bristol Myers Squibb, and consulting fees for PNH Registry from Alexion Pharmaceuticals; reports payment or honoraria for an online debate from Novartis; and reports participation on a data safety monitoring board or advisory board from Omeros. B.S. reports support for the present manuscript for participation in the Delphi panel outlined in the manuscript by Novartis; reports consulting fees from Bristol Myers Squibb, Alexion, Novartis, and Incyte; reports payment or honoraria from Bristol Myers Squibb, Alexion, Novartis, and Jazz; reports participation on a data safety monitoring board or advisory board from Nektar, Alexion, and Bristol Myers Squibb; and reports leadership or fiduciary role in other board, society, committee, or advocacy group paid or unpaid from ASH Government Affairs. S.T. reports support for the present manuscript for participation in the Delphi panel outlined in the manuscript by Novartis; reports grants or contracts from Karyopharm Therapeutics Inc as an investigator who initiated clinical trial and preclinical studies; reports consulting fees from consulting/advisory board from Karyopharm Therapeutics Inc, Novartis, AbbVie, MorphoSys, and CTI Biopharma; and reports support for attending meetings and/or travel from Karyopharm Therapeutics for 2023 AACR meeting. M.W.W. reports support for the present manuscript for participation in the Delphi panel outlined in the manuscript by Novartis. I.Y. is an employee of Partnership for Health Analytic Research, which was paid by Novartis to conduct the research described in this manuscript, and by Akcea, Amgen, BioMarin Pharmaceuticals, Bristol Myers Squibb, Celgene, Delfi Diagnostics, Dompe, Eisai, Exact Sciences Corporation, Genentech, Gilead, GRAIL, Greenwich Biosciences, Ionis, Jazz, Nobelpharma, Novartis, Otsuka, Pfizer, Recordati, Regeneron, Sanofi US Services, and Takeda Pharmaceuticals USA to conduct research related to the work described in this manuscript and outside of this submitted work. B.J.P. reports support for the present manuscript for participation in the Delphi panel outlined in the manuscript by Novartis and to help write the manuscript, participation as the panel chair, being an active panelist and working on the manuscript with Novartis (note this was a blinded sponsored project, and payment was made to B.J.P.); and reports payment for training and education on Promacta with Novartis (payment was made to B.J.P.).
Correspondence: Bhumika J. Patel, Prisma Health, 701 Grove Rd, Greenville, SC 29605; email: bhumika.patel@prismahealth.org.
References
Author notes
A copy of the rating form may be found in the data supplement available with the online version of this article.
The full-text version of this article contains a data supplement.