Key Points
PDK2/4 reduces susceptibility to DVT by limiting NETosis.
PDK2/4 potentiates NETosis by increasing intracellular calcium concentration, Erk 1/2 phosphorylation, and aerobic glycolysis.
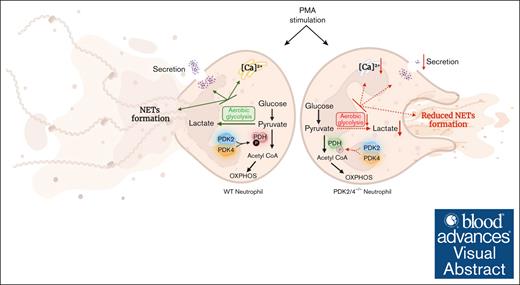
Visual Abstract
Neutrophils contribute to deep vein thrombosis (DVT) by releasing prothrombotic neutrophil extracellular traps (NETs). NET formation (known as NETosis) is an energy-intensive process that requires an increased rate of aerobic glycolysis. The metabolic enzymes pyruvate dehydrogenase kinases (PDKs) inhibit the pyruvate dehydrogenase complex to divert the pyruvate flux from oxidative phosphorylation toward aerobic glycolysis. Herein, we identified that the combined deletion of PDK2 and PDK4 (PDK2/4–/–) renders mice less susceptible to DVT (measured by thrombus incidence, weight, and length) in the inferior vena cava–stenosis model at day 2 after surgery. Compared with wild-type (WT) mice, the venous thrombus obtained from PDK2/4–/– mice exhibited reduced citrullinated histone content, a known marker of NETs. In line with in vivo observations, phorbol 12-myristate 13-acetate (PMA)–stimulated PDK2/4–/– neutrophils displayed reduced NETosis and secretion of cathepsin G and elastase compared with PMA-stimulated WT neutrophils. The formation of platelet aggregates mediated by PMA-stimulated PDK2/4–/– neutrophils were significantly reduced compared with PMA-stimulated WT neutrophils. Finally, PDK2/4–/– neutrophils exhibited reduced levels of intracellular Ca2+ concentration, extracellular signal-regulated kinase 1/2 (Erk1/2) phosphorylation, and glycolytic proton efflux rate (a measure of aerobic glycolysis), known to facilitate NETosis. Together, these findings elucidate, to our knowledge, for the first time, the fundamental role of PDK2/4 in regulating NETosis and acute DVT.
Introduction
Deep vein thrombosis (DVT) and its major acute complication, pulmonary embolism, are associated with substantial mortality and long-term disability worldwide.1 Although prophylactic use of anticoagulant therapy remains the cornerstone for preventing DVT, the associated bleeding risk considerably limits its long-term use.2 The initiation and progression of DVT involves a combination of factors, including a hypercoagulation state; disturbed venous flow; and the activation of endothelium, platelets, and leukocytes.3 Among leukocyte subtypes, neutrophils represent the most prominent contributors to DVT initiation.4 After recruitment to the activated endothelium lining of the vein (caused by local hypoxia due to disrupted blood flow), neutrophils release their DNA to form neutrophil extracellular traps (NETs), a process known as NETosis.5,6 NETs promote the coagulation cascade and provide a scaffold for prothrombotic molecules (such as von Willebrand factor, fibronectin, and fibrinogen) and facilitate platelet activation, ultimately leading to the development of DVT.4,7
Recent bioenergetic analysis suggests that neutrophil activation and NET formation is an energy-intensive process, characterized by an increased rate of aerobic glycolysis (lactate formation in the presence of oxygen, also known as the Warburg effect).8 The pyruvate dehydrogenase (PDH) complex converts pyruvate (the end product of glycolysis) to acetyl coenzyme A, which is further oxidized via the Krebs cycle to produce adenosine triphosphate (ATP) using mitochondrial oxidative phosphorylation (OXPHOS).9 The activity of the PDH complex is tightly regulated by pyruvate dehydrogenase kinases (PDKs), which phosphorylate the PDH complex to inhibit its activity, thereby diverting the pyruvate flux from OXPHOS toward aerobic glycolysis (lactate formation). Therefore, the PDK/PDH axis is regarded as a vital metabolic checkpoint that regulates the pyruvate flux and aerobic glycolysis in activated neutrophils. Four isozymes of PDKs (PDK1-4) exist in humans and rodents, of which PDK2 and PDK4 (PDK2/4) exhibit ubiquitous tissue distribution and are prominently associated with metabolic diseases, compared with PDK1 and PDK3.10 We recently reported that genetic deletion of PDK2/4 inhibits aerobic glycolysis in platelets, leading to reduced platelet activation and arterial thrombosis without altering hemostasis.11 The role of PDK2/4 in NET formation and DVT remains unexplored. Herein, we sought to investigate the regulatory role of PDK2/4 in NETosis and acute DVT.
Materials and methods
Materials
Prostaglandin I2 (PGI2), phorbol 12-myristate 13-acetate (PMA), and Ponceau S were purchased from Sigma-Aldrich. Super Signal West Pico chemiluminescent substrate, Sytox green, Hoechst, Fura 2-acetoxymethyl ester (Fura2-AM), and probenecid were from Thermo Fisher. The сathepsin G enzyme-linked immunosorbent assay kit was purchased from LSBio (LS-F7218). The elastase enzyme-linked immunosorbent assay kit was from R&D Systems (MELA20). Primary antibodies anti-citrullinated H3 was purchased from Abcam (catalog no. ab5103), and phospho-p44/42 MAPK (extracellular signal-regulated kinase 1/2 [Erk1/2]; Thr202/Tyr204; clone D13.14.4E, catalog no. 4370) and p44/42 MAPK (Erk1/2; clone 137F5, catalog no. 4695) were purchased from Cell Signaling Technologies. PDK2 (clone S-15, catalog no. Sc100534) was purchased from Santacruz; PDK4 (catalog no. 1249-1-AP) was purchased from ProteinTech. The TruStain FcX PLUS anti-mouse CD16/CD32 (clone S17011E, catalog no. 156604) and Alexa Fluor 647 anti-mouse Ly6G (clone 1A8, catalog no. 127610) antibodies were purchased from BioLegend. Fluorescein isothiocyanate anti-mouse CD41/CD61 (clone Leo.F2, catalog no. M025-1) antibody was purchased from Emfret Analytics. All other reagents were of analytical grade.
Mice
We generated wild-type (WT; PDK2/4+/+) and global double-knockout (PDK2/4–/–) from heterozygous (PDK2+/–PDK4+/–) mice.11 The mice used in the study were males, aged between 10 and 12 weeks, and on the C57BL/6J background. Female mice were not used in the study because of existing evidence that they are less susceptible to acute DVT because of differences in sex-specific growth hormone as well as uncompromised uterine venous flow to the inferior vena cava (IVC) that may limit thrombi progression and may confound the findings.12 The mice were kept in standard animal housing conditions with controlled temperature and humidity and had ad libitum access to a standard chow diet and water. The University of Iowa Animal Care and Use Committee approved all experiments.
IVC-stenosis model for DVT
DVT was induced using a validated model of venous stenosis (flow restricted), and IVC ligation, which is characterized by a closure of ≥90% of the IVC lumen.13 Briefly, mice were anesthetized, and the IVC separated from the aorta, just inferior to the renal veins. The IVC stenosis was performed by applying a provisional knot with the 5-0 prolene suture (but not fully closed) and by placement of a 30-gauge needle (“a spacer”) bent as a hook into the knot. After positioning the spacer inside the knot, it was tightly closed, and the spacer was removed. All visible side branches were ligated with 5-0 prolene sutures. Mice were then allowed to recover and later euthanized 48 hours after surgery. The length and weight of the thrombi isolated from the vessel were measured immediately after isolation. As required, the thrombus was processed for protein estimation for western blotting.
Mouse platelet isolation
The mice were bled from the retro-orbital plexus in 1.5-mL tubes containing enoxaparin. The washed platelets were prepared, as explained previously.14,15 Briefly, the whole blood was centrifuged at 100g for 5 minutes at room temperature (RT). Next, the supernatant containing platelet-rich plasma was transferred to a fresh tube and incubated in PGI2 (2 μg/mL) at 37°C for 5 minutes to prevent platelet activation. Next, the platelet-rich plasma was centrifuged at 600g for 5 minutes at RT; the pellet was then rinsed and resuspended in 1× modified Tyrodes buffer (1 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 137 mM NaCl, 0.3 mM Na2HPO4, 2 mM KCl, 12 mM NaHCO3, 5 mM N-2-hydroxyethylpiperazine-N′-2-ethane sulfonic acid, 5 mM glucose, and 0.35% bovine serum albumin; pH 7.2) containing PGI2 (2 μg/mL). The rinsing was repeated with Tyrodes buffer in the absence of PGI2, followed by final resuspension in 1× Tyrodes buffer.
NETosis assay
Freshly isolated neutrophils from the bone marrow were seeded on poly-L-lysine–coated coverslips (1 × 104 cells per coverslip). Cells were incubated for 60 minutes in a CO2 incubator. NETosis assay was performed by stimulating cells with PMA (10 nM) and incubating for 4 hours in a CO2 incubator at 37°C. Ice-cold phosphate-buffered saline (PBS) was added to stop the reaction. The cells were fixed in ice-cold PBS containing 2% paraformaldehyde. The fixed cells were then washed with ice-cold PBS. For specific staining of extracellular nuclear structures and nucleus, cells were incubated at RT with Sytox Green dye and Hoechst, respectively. Coverslips were washed with PBS and mounted onto glass slides using a drop of mounting medium (ProLong Gold Antifade Mountant, ThermoFisher), before fluorescence microscopy analysis. Two different fields at 20× magnification were used to estimate the number of neutrophils releasing NETs, which was expressed as a percentage of total neutrophils (coverslip edges were avoided).
Glycolytic rate assay
The neutrophils were diluted to a concentration of 4 × 105 cells in XF Dulbecco’s modified Eagle medium assay buffer (Dulbecco’s modified Eagle medium with 1 mM pyruvate, 5.5 mM D-glucose, 4 mM L-glutamine; pH 7.4) and seeded onto Cell-Tak–coated XF96 microplates. The 96-well format Seahorse extracellular flux analyzer, XF96, was used to measure the glycolytic proton efflux rate (glycoPER) in resting and PMA-stimulated (100 nM) WT and PDK2/4–/– neutrophils.11,15,16
Neutrophil–induced platelet aggregation
Platelets and neutrophils from either WT or PDK2/4–/– mice were coincubated to obtain a final concentration of 4 × 108 platelets per mL and 1.2 × 107 neutrophils per mL in Tyrodes buffer. This platelet–neutrophil suspension was stirred at 1200 rpm and 37°C for 20 minutes in a Chrono-log Whole-Blood/Optical Lumi-Aggregometer before the addition of PMA (10 nM). Aggregation was measured as the percent change in light transmission, with 100% referring to the transmittance of light through the blank sample.
Neutrophil-platelet complex assay
The bone marrow–derived WT or PDK2/4–/– neutrophils (1.2 × 107 cells per mL) were coincubated with WT washed platelets (4 × 108 cells per mL) on a rocking platform. The cells were blocked with Fc-block (anti-mouse CD16/CD32), then stained with Alexa Fluor 647 anti-mouse Ly6G, and fluorescein isothiocyanate anti-mouse CD41/CD61 antibodies before their stimulation with PMA (10 nM) at 37°C for 15 minutes by gentle shaking (30 rpm) on a rocking platform. The small neutrophil–platelet complexes were defined as CD41 and Ly6G double-positive clusters using the LSR II Flow Cytometer (BD Biosciences). Data were analyzed using FlowJo cell analysis software.
Intracellular Ca2+ concentration measurement
The neutrophils (5 × 106 cells per mL) were loaded with 5 μM of Fura2-AM and 4 mM probenecid at RT for 45 minutes for measuring intracellular Ca2+ concentration. The neutrophils were then centrifuged at 600g for 5 minutes to remove the excessive extracellular dye. The neutrophils were resuspended in Hanks balanced salt solution (HBSS) without Ca2+. Neutrophils were stimulated with PMA (2 nM) in the presence of 1.3 mM CaCl2. The intracellular Ca2+ concentration was measured using a fluorometer at 340 and 380 nm excitation, and 509 nm emission for Fura2-AM. The intracellular Ca2+ concentration was calculated using the following formula [Ca2+] = Kdβ(R−Rmin)/(Rmax−R); in which R = F340/F380, Rmax and Rmin are F340/F380 fluorescence ratios determined for Ca2+-bound and Ca2+-free forms of Fura2-AM, respectively. Kd is Ca2+ dissociation constant of Fura2-AM, and β = Fmin,380/Fmax,380, in which Fmax,380 and Fmin,380 are fluorescence intensities for excitation at 380 nm determined for Ca2+-bound and Ca2+-free forms of Fura2-AM, respectively. The Fmin was obtained by quenching the signal by adding 4 mM EGTA. The Fmax was acquired by lysing the cells with Trion X-100.
Cathepsin G and elastase release assay
Cathepsin G and elastase in the supernatant from PMA-stimulated neutrophils were measured using the commercially available kit as per the manufacturer’s protocol.
Western blot analysis
Neutrophil proteins were separated on 4% to 20% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gradient gels and electrophoretically transferred to polyvinylidene fluoride membrane by using Bio-Rad western blotting system. Membranes were blocked with 5% bovine serum albumin in 10 mM Tris-HCl, 150 mM NaCl, pH 8.0 (Tris-buffered saline) containing 0.05% Tween-20 for 1 hour at RT. Blots were incubated overnight with the primary antibody, followed by horseradish peroxidase–labeled secondary antibody for 1 hour. Blots were developed using enhanced chemiluminescence and quantified using Image J software.
Statistical analysis
Data are represented as mean ± standard error of the mean (SEM). The statistical significance involving 2 groups was assessed using a Mann-Whitney U test. For >2 groups, significance was determined by 1-way analysis of variance or 2-way analysis of variance followed by Tukey or Šidák multiple comparisons tests. P < .05 was considered to be statistically significant. GraphPad Prism software version 10 was used for the statistical analysis.
Results
Double-deficient PDK2/4 mice are less susceptible to acute DVT
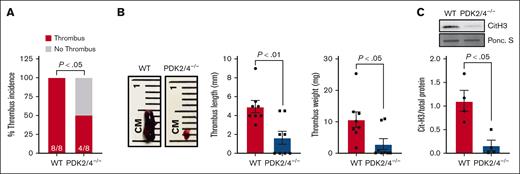
We confirmed the deletion of PDK2/4 from neutrophils by western blotting (supplemental Figure 1A). The body weight and complete blood count were comparable between the PDK2/4–/– and WT mice.11 To determine the effects of combined deletion of PDK2/4 on venous thrombosis, we evaluated the susceptibility of PDK2/4–/– male mice to acute DVT using the IVC-stenosis model. Compared with WT mice, the deletion of PDK2/4 significantly reduced susceptibility to acute DVT, as evidenced by lower thrombus incidence (50% vs 100%), length, and weight on day 2 after surgery (Figure 1A,B). The intrinsic coagulation pathway is known to contribute to the pathogenesis of venous thrombosis.17 To determine whether the global PDK2/4 deletion affects clotting factors and ex vivo coagulation, we measured the plasma recalcification time and found it to be comparable between the WT and PDK2/4–/– mice, suggesting a normal intrinsic coagulation pathway (supplemental Figure 1B).
The combined deletion of PDK2/4 inhibits acute DVT. (A) Thrombus incidence in male PDK2/4–/– mice and WT mice 48 hours after IVC stenosis; n = 8 mice per group. Statistical analysis was performed using Fisher exact test. (B) The representative image of IVC thrombus harvested from PDK2/4–/– mice and WT mice 48 hours after IVC stenosis. The bar graphs show quantified mean thrombus weight and thrombus length. Values are mean ± SEM, n = 8 mice per group. Statistical analysis was performed using Mann-Whitney U test. (C) The level of Cit-H3 was measured in the thrombus isolated from PDK2/4–/– mice and WT mice 48 hours after IVC stenosis. A representative western blot for Cit-H3 is shown. Ponceau S (Ponc. S) staining was used as a loading control. The bar graphs show densitometry analysis of immunoblots. Values are mean ± SEM, with n = 4 mice per group. Statistical analysis was performed using Mann-Whitney U test.
The combined deletion of PDK2/4 inhibits acute DVT. (A) Thrombus incidence in male PDK2/4–/– mice and WT mice 48 hours after IVC stenosis; n = 8 mice per group. Statistical analysis was performed using Fisher exact test. (B) The representative image of IVC thrombus harvested from PDK2/4–/– mice and WT mice 48 hours after IVC stenosis. The bar graphs show quantified mean thrombus weight and thrombus length. Values are mean ± SEM, n = 8 mice per group. Statistical analysis was performed using Mann-Whitney U test. (C) The level of Cit-H3 was measured in the thrombus isolated from PDK2/4–/– mice and WT mice 48 hours after IVC stenosis. A representative western blot for Cit-H3 is shown. Ponceau S (Ponc. S) staining was used as a loading control. The bar graphs show densitometry analysis of immunoblots. Values are mean ± SEM, with n = 4 mice per group. Statistical analysis was performed using Mann-Whitney U test.
PMA-stimulated PDK2/4–/– neutrophils are less efficient in inducing NETosis
The formation of NETs plays a fundamental role in the onset and development of DVT. To assess the impact of PDK2/4 deletion on NET formation in vivo, we quantified citrullinated histones (Cit-H3), a marker of NETs, in thrombi obtained from WT and PDK2/4–/– mice after IVC stenosis. Compared with WT mice, thrombi from PDK2/4–/– mice exhibited a significantly reduced presence of Cit-H3, suggesting reduced NETs content in the venous thrombus (Figure 1C). To investigate the effect of PDK2/4 deletion on NETosis further, we stimulated bone marrow–derived neutrophils from WT and PDK2/4–/– mice with PMA, a well-characterized NETosis inducer.18 Consistent with the reduced content of Cit-H3 in thrombi from PDK2/4–/– mice, the release of NETs from PDK2/4–/– neutrophils was significantly attenuated compared with WT neutrophils after PMA stimulation (Figure 2).
PDK2/4–/– neutrophils exhibit reduced release of NETs. NETs assay was performed by stimulating bone marrow–derived WT and PDK2/4–/– neutrophils with PMA (10 nM) for 4 hours. Representative microphotographs of NETs stained with Sytox green (stains extracellular DNA, green) and counterstained with Hoechst (stains nuclei, blue) are shown. The bar graph shows the quantification of the percentage of cells releasing NETs. Values are mean ± SEM, n = 5 mice per group. Two different fields at 20× magnification were used to estimate the number of neutrophils releasing NETs, which was expressed as a percentage of total neutrophils (coverslip edges were avoided). Statistical analysis was performed using 2-way analysis of variance (ANOVA) followed by Tukey multiple comparisons test.
PDK2/4–/– neutrophils exhibit reduced release of NETs. NETs assay was performed by stimulating bone marrow–derived WT and PDK2/4–/– neutrophils with PMA (10 nM) for 4 hours. Representative microphotographs of NETs stained with Sytox green (stains extracellular DNA, green) and counterstained with Hoechst (stains nuclei, blue) are shown. The bar graph shows the quantification of the percentage of cells releasing NETs. Values are mean ± SEM, n = 5 mice per group. Two different fields at 20× magnification were used to estimate the number of neutrophils releasing NETs, which was expressed as a percentage of total neutrophils (coverslip edges were avoided). Statistical analysis was performed using 2-way analysis of variance (ANOVA) followed by Tukey multiple comparisons test.
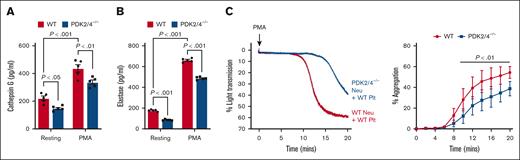
The combined deletion of PDK2/4 results in reduced neutrophil-mediated platelet aggregation
The in vivo findings suggest that PDK2/4 contributes to acute DVT, most likely by promoting NETosis. Next, we examined the underlying mechanisms by which PDK2/4 may contribute to NETosis and thereby to the progression of acute DVT. It is known that neutrophil–platelet interactions play a pivotal role in the development of acute DVT.19 This interaction generates a positive feedback loop that amplifies NETosis and further reinforces thrombus progression.20 To dissect this axis, we examined the secretome of activated WT and PDK2/4–/– neutrophils by quantifying cathepsin G and elastase, known to activate platelets through protease-activated receptors.21 We found that there was a significant reduction in the release of cathepsin G (Figure 3A) and elastase (Figure 3B) from PMA-stimulated PDK2/4–/– neutrophils compared with PMA–stimulated WT neutrophils. Thereafter, we examined the effects of reduced secretion from PMA-stimulated PDK2/4–/– neutrophils on platelet aggregation. The WT platelets that were incubated with PDK2/4–/– neutrophils displayed reduced aggregation compared with WT platelets incubated with WT neutrophils, after the stimulation of neutrophils with PMA (Figure 3C). Although we have used a suboptimal concentration of PMA (10 nM), 1 of the limitations of this assay is that PMA is known to activate platelets in addition to neutrophils.22 Next, to rule out the effect of PDK2/4 deficiency in platelets on neutrophil-mediated aggregation, we compared the extent of aggregation between WT and PDK2/4–/– platelets coincubated with PMA–stimulated WT neutrophils. The extent of aggregation was comparable between WT and PDK2/4–/– platelets (supplemental Figure 2A). To examine whether neutrophil PDK2/4 also promote the formation of small platelet–neutrophil complexes, WT platelets were coincubated with PDK2/4–/– or WT neutrophils under non-stirring condition and stimulated with the same concentration of PMA (10 nM), as used for platelet aggregation assay. Flow cytometry analysis revealed that the extent of small platelet–neutrophil complexes formed by PDK2/4–/– neutrophils with WT platelets was comparable with that of WT neutrophils (supplemental Figure 2B).
Combined deletion of PDK2/4 results in reduced neutrophil-mediated platelet aggregation. The extent of (A) cathepsin G and (B) elastase release was measured using enzyme-linked immunosorbent assay in WT or PDK2/4–/– neutrophils that were stimulated with PMA (50 nM) for 30 minutes. Values are mean ± SEM, n = 5 mice per group. Statistical analysis was performed using 2-way ANOVA followed by Tukey multiple comparisons test. (C) WT platelets were coincubated and stirred with WT or PDK2/4–/– neutrophils in an aggregometer before the addition of PMA (10 nM). Results are expressed as the percent change in light transmission with respect to the blank (buffer without platelets and neutrophils) set at 100%. Left: the representative aggregation curve is shown. Right: the line graph shows the quantified data for platelet aggregation. Values are mean ± SEM, n = 5 mice per group. Statistical analysis was performed using 2-way ANOVA followed by Šidák multiple comparisons test. Neu, neutrophils; Plt, platelets.
Combined deletion of PDK2/4 results in reduced neutrophil-mediated platelet aggregation. The extent of (A) cathepsin G and (B) elastase release was measured using enzyme-linked immunosorbent assay in WT or PDK2/4–/– neutrophils that were stimulated with PMA (50 nM) for 30 minutes. Values are mean ± SEM, n = 5 mice per group. Statistical analysis was performed using 2-way ANOVA followed by Tukey multiple comparisons test. (C) WT platelets were coincubated and stirred with WT or PDK2/4–/– neutrophils in an aggregometer before the addition of PMA (10 nM). Results are expressed as the percent change in light transmission with respect to the blank (buffer without platelets and neutrophils) set at 100%. Left: the representative aggregation curve is shown. Right: the line graph shows the quantified data for platelet aggregation. Values are mean ± SEM, n = 5 mice per group. Statistical analysis was performed using 2-way ANOVA followed by Šidák multiple comparisons test. Neu, neutrophils; Plt, platelets.
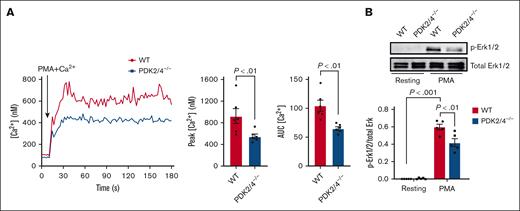
Combined PDK2/4 deletion inhibits the intracellular Ca2+ levels in PMA-stimulated neutrophils
The rise in the intracellular Ca2+ levels and the phosphorylation of Erk1/2 are critical molecular events underlying PMA-induced neutrophil activation and the release of NETs.23,24 Compared with WT neutrophils, we observed a significant reduction in intracellular Ca2+ levels in PDK2/4–/– neutrophils after PMA stimulation (Figure 4A). The reduced intracellular Ca2+ concentration was associated with decreased Erk1/2 phosphorylation in PDK2/4–/– neutrophils compared with WT neutrophils after PMA stimulation (Figure 4B). These findings suggest an essential role of PDK2/4 in regulating Ca2+ signaling and the Erk1/2 pathway during NETosis.
Double deletion of PDK2/4 inhibits intracellular Ca2+ concentration levels in PMA-stimulated neutrophils. (A) The intracellular Ca2+ concentration was measured in Fura-2AM–loaded WT or PDK2/4−/− neutrophils that were stimulated with PMA (2 nM) in the presence of 1.3 mM CaCl2. The representative curve for intracellular calcium Ca2+ concentration after stimulation with PMA is shown. The bar graphs show the peak Ca2+ level and the area under the curve (AUC). Values are mean ± SEM, n = 6 mice per group. Statistical analysis was performed using Mann-Whitney U test. (B) The Erk1/2 phosphorylation was measured in resting and PMA (50 nM)–stimulated WT and PDK2/4−/− neutrophils. A representative western blot for phosphorylated Erk1/2 is shown. Total Erk1/2 was used as a loading control. The bar graphs show densitometry analysis of immunoblots. Values are mean ± SEM, n = 5 mice per group. Statistical analysis was performed using 2-way ANOVA followed by Tukey multiple comparisons test.
Double deletion of PDK2/4 inhibits intracellular Ca2+ concentration levels in PMA-stimulated neutrophils. (A) The intracellular Ca2+ concentration was measured in Fura-2AM–loaded WT or PDK2/4−/− neutrophils that were stimulated with PMA (2 nM) in the presence of 1.3 mM CaCl2. The representative curve for intracellular calcium Ca2+ concentration after stimulation with PMA is shown. The bar graphs show the peak Ca2+ level and the area under the curve (AUC). Values are mean ± SEM, n = 6 mice per group. Statistical analysis was performed using Mann-Whitney U test. (B) The Erk1/2 phosphorylation was measured in resting and PMA (50 nM)–stimulated WT and PDK2/4−/− neutrophils. A representative western blot for phosphorylated Erk1/2 is shown. Total Erk1/2 was used as a loading control. The bar graphs show densitometry analysis of immunoblots. Values are mean ± SEM, n = 5 mice per group. Statistical analysis was performed using 2-way ANOVA followed by Tukey multiple comparisons test.
PMA-stimulated PDK2/4–/– neutrophils exhibit reduced aerobic glycolysis
To elucidate the fundamental role of PDKs in modulating aerobic glycolysis in neutrophils, we performed a glycolytic rate assay to measure the glycoPER, a measure of aerobic glycolysis (and lactic acid production). In live cells, glycolysis-derived lactic acid and mitochondrial OXPHOS-derived CO2 are the 2 primary sources of extracellular protons, which together constitute the proton efflux rate. GlycoPER is determined by treating cells with rotenone and antimycin A to inhibit mitochondrial function and thus eliminate mitochondrial CO2-mediated acidification.15,25 After PMA stimulation, the extent of proton efflux rate and glycoPER was significantly reduced in PDK2/4−/− neutrophils compared with WT neutrophils (Figure 5), suggesting a negative regulation of aerobic glycolysis and lactic acid production in activated PDK2/4−/− neutrophils. These findings provide mechanistic evidence for the role of the PDK2/4 in promoting NETosis and, consequently, DVT, most likely through the modulation of aerobic glycolysis in neutrophils.
Deletion of PDK2/4 inhibits aerobic glycolysis in PMA-stimulated neutrophils. The proton efflux rate (PER) and glycoPER were measured in PMA (100 nM)–stimulated WT or PDK2/4−/− neutrophils using glycolytic rate assay. The line graph represents the PER, and compensatory glycoPER (after the sequential addition of mitochondrial inhibitors, rotenone and antimycin A [AA]). The middle and right panels show the quantification of PER and glycoPER. The bar graph shows the quantified data. Values are mean ± SEM, with n = 4 to 5 mice per group. Statistical analysis was performed using 2-way ANOVA followed by Tukey multiple comparisons test.
Deletion of PDK2/4 inhibits aerobic glycolysis in PMA-stimulated neutrophils. The proton efflux rate (PER) and glycoPER were measured in PMA (100 nM)–stimulated WT or PDK2/4−/− neutrophils using glycolytic rate assay. The line graph represents the PER, and compensatory glycoPER (after the sequential addition of mitochondrial inhibitors, rotenone and antimycin A [AA]). The middle and right panels show the quantification of PER and glycoPER. The bar graph shows the quantified data. Values are mean ± SEM, with n = 4 to 5 mice per group. Statistical analysis was performed using 2-way ANOVA followed by Tukey multiple comparisons test.
Discussion
Although neutrophils are well known to derive most of their energy from glycolysis (given the limited mitochondrial density),26 the presence of aerobic glycolysis as a metabolic pathway driving neutrophil activation and NETosis has only recently been identified.8 In this study, we provide evidence that targeting PDK2/4 inhibits NET formation by reducing aerobic glycolysis, which further prevents DVT incidence and burden. Although considered less efficient, aerobic glycolysis facilitates rapid ATP synthesis (100× faster compared with OXPHOS),27 which suits the immediate energy requirement of neutrophil activation and the release of NETs. In addition to neutrophils, increasing evidence suggests that switching to aerobic glycolysis for ATP synthesis is a conserved metabolic feature shared by a range of blood cells including platelets, known to support DVT progression.28 Notably, we previously reported that the pharmacological targeting of PDKs or genetic deletion of PDK2/4 inhibits platelet activation and arterial thrombosis, primarily through the attenuation of aerobic glycolysis.11,16 Furthermore, the deletion of PDK2/4 has also been reported to alleviate acute inflammation, followed by reduced neutrophil infiltration at the site of inflammation.29 Collectively, these previous results, along with our current findings, imply that targeting PDKs may offer synergistic protection against acute DVT by inhibiting platelet activation, inflammation, and NETosis.
The development of DVT requires sustained cooperation between neutrophils and platelets that is orchestrated directly through receptor-mediated interaction and indirectly through the release of granule-derived soluble mediators from both cell types.30 Herein, we observed that reduced aerobic glycolysis was associated with impaired secretion from PMA-activated PDK2/4–/– neutrophils, which further resulted in an inhibition of WT platelet aggregation. In contrast, we did not observe any effect on PDK2/4–/– platelet aggregation upon activation with PMA-stimulated WT neutrophils. Although interesting, this observation is not entirely surprising because activated neutrophils release a variety of molecules such as cathelicidins, elastase, cathepsin G, and reactive oxygen species that are known to activate platelets.30 Thus, the concurrent and synergistic effects of these molecules may overcome the inhibitory effects of PDK2/4 deletion in platelets exhibiting a comparable aggregation response to WT platelets. Furthermore, intracellular Ca2+ and the Erk1/2 pathway play a pivotal role in facilitating PMA-induced NETosis.23,31 PMA directly activates the protein kinase C, which leads to Ca2+ fluxes in neutrophils, leading to NETosis32; whereas the Erk1/2 pathway reportedly promotes the expression of antiapoptotic proteins in neutrophils, which delay apoptosis and subsequently amplify NETosis.24 Consistent with the impaired release of NETs, we observed that the rise in intracellular Ca2+ concentration and the level of Erk1/2 phosphorylation in PMA-stimulated PDK2/4–/– neutrophils was significantly reduced.
Although our study suggests a key role of PDK2/4 in regulating NETosis and DVT, the role of PDK1 and PDK3 cannot be ruled out and requires further investigation using transgenic mouse models. One of the limitations of this study is that we have used global PDK2/4−/− mice, and not the neutrophil-specific PDK-null mice (for all the isoforms) given their unavailability. Therefore, further studies are warranted to dissect the neutrophil-specific contributions of PDKs in the development of DVT. Furthermore, given the important role of platelets in the development of DVT, it is also important to develop PDK-floxed mice to determine the platelet-specific contribution of PDKs toward DVT.
In summary, the data presented here suggest that targeting PDKs in neutrophils can offer a broad range of protective effects against DVT, primarily by inhibiting aerobic glycolysis and NET formation (Figure 6). However, such an approach would need to be comprehensively evaluated clinically for potential bleeding risks. Notably, we did not observe any defects in hemostasis during the pharmacological or genetic targeting of PDKs in vivo.11,16
A schematic depicting the role of the PDK/PDH axis in regulating neutrophil activation and NET formation. The deletion of PDK2/4 inhibits aerobic glycolysis, secretion, and intracellular Ca2+ levels in PMA-stimulated neutrophils, leading to reduced release of NETs.
A schematic depicting the role of the PDK/PDH axis in regulating neutrophil activation and NET formation. The deletion of PDK2/4 inhibits aerobic glycolysis, secretion, and intracellular Ca2+ levels in PMA-stimulated neutrophils, leading to reduced release of NETs.
Acknowledgments
The A.K.C. laboratory is supported by grants from the National Institutes of Health, the National Heart, Lung, and Blood Institute (R35HL139926), and the National Institute of Neurological Disorders and Stroke (R01NS109910 and U01NS130587).
The figures were created using BioRender.com.
Authorship
Contribution: G.D.F. and M.G. performed experiments and analyzed the data; M.K.N., T.B., and M.K. performed experiments; G.D.F. and A.K.C. wrote the original draft of the manuscript; and all authors reviewed and edited the manuscript.
Conflict-of-interest: The authors declare no competing financial interests.
Correspondence: Gagan D. Flora, Department of Internal Medicine, University of Iowa, 3160 Medical Laboratories, Iowa City, IA 52242; email: gagandeep-flora@uiowa.edu; and Anil K. Chauhan, Department of Internal Medicine, University of Iowa, 3160 Medical Laboratories, Iowa City, IA 52242; email: anil-chauhan@uiowa.edu.
References
Author notes
Data are available on reasonable request from the corresponding authors, Gagan D. Flora (gagandeep-flora@uiowa.edu) and Anil K. Chauhan (anil-chauhan@uiowa.edu).
The full-text version of this article contains a data supplement.


![Deletion of PDK2/4 inhibits aerobic glycolysis in PMA-stimulated neutrophils. The proton efflux rate (PER) and glycoPER were measured in PMA (100 nM)–stimulated WT or PDK2/4−/− neutrophils using glycolytic rate assay. The line graph represents the PER, and compensatory glycoPER (after the sequential addition of mitochondrial inhibitors, rotenone and antimycin A [AA]). The middle and right panels show the quantification of PER and glycoPER. The bar graph shows the quantified data. Values are mean ± SEM, with n = 4 to 5 mice per group. Statistical analysis was performed using 2-way ANOVA followed by Tukey multiple comparisons test.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/15/10.1182_bloodadvances.2024013199/2/m_blooda_adv-2024-013199-gr5.jpeg?Expires=1769235596&Signature=Zdu1i-qgTqfcG2XEsAWI4u5MSn-CvyA1TCctMlWWI3hc88-8rpimOIV8yPJSBoS~krIZGcGOni0t7bGlk8eZpko1nL1BwhCSMjb0OMGXKNzWPFrbvqkjmgkhae8D~ERSafSH27uNqWJd4iGbikk6I5LJjNyhGgxe8sRrjauCVbk6zuzuZN00ciSE2DPctrXr8cjFIeQ3WfvxaLq~P61GuafTuebFacOZ9htzMTCXTszm572nr0TJgW2gHyWuBRFOclkPyRowClShoqJeCEO5DoGzT45cGNhUx4Aie1phGZTYbA2q0Cq7ABv~eZqtqZoud0X535AbR2tYwb4QHvc2mA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

