Key Points
In pregnant women, physiological ferritin thresholds for ID are ∼25 μg/L in the first and ∼20 μg/L in the second and third trimesters.
These physiologically identified ferritin thresholds for ID track concentrations of hepcidin, the iron-regulatory hormone.
Visual Abstract
Serum ferritin (SF) concentration is the most widely used indicator for iron deficiency (ID). During pregnancy, the World Health Organization recently recommended SF thresholds for ID of <15 μg/L for the first trimester of pregnancy, based on expert opinion, and made no recommendations for the second and third trimesters. We examined the relationship of SF with 2 independent indicators of the onset of iron-deficient erythropoiesis, hemoglobin and soluble transferrin receptor 1, in cross-sectional data from US National Health and Nutrition Examination Survey for 1999 to 2010 and 2015 to 2018. We included 1288 pregnant women aged 15 to 49 years and excluded women with inflammation or potential liver disease. We used restricted cubic spline (RCS) regression analysis to determine SF thresholds for iron-deficient erythropoiesis. SF decreased during pregnancy; geometric mean SF was higher during the first and lower during the second and third trimesters. Using RCS analysis, the SF thresholds identified during pregnancy were <25.8 μg/L (18.1-28.5) during first trimester, <18.3 μg/L (16.3-22.9) during second trimester, and <19.0 μg/L (14.4- 26.1) during third trimester. These SF threshold levels track concentrations of hepcidin, the iron-regulatory hormone controlling the mobilization of iron stores. An SF concentration of <15 μg/L as the criterion for ID may underestimate the true prevalence of ID throughout pregnancy. In our study, an additional 1 of every 10 pregnant women would be recognized as iron deficient by using the physiologically based thresholds at SF of ∼25 μg/L during the first and ∼20 μg/L during the second and third trimesters.
Introduction
The goal of our study is to extend to apparently healthy pregnant women a physiologically based method to determine serum ferritin (SF) concentration thresholds for iron deficiency (ID). ID in pregnancy is a vital public health problem associated with harmful health outcomes in the mother and child.1-6 In earlier studies of children and of women of reproductive age who were not pregnant, we developed a method to examine 2 physiological indicators of ID, hemoglobin (Hb) and soluble transferrin receptor (sTfR1) concentrations, to determine SF thresholds for ID.7-9 With the development of ID in otherwise healthy, nonpregnant women, because the supply of iron becomes insufficient for Hb synthesis, the onset of iron-restricted erythropoiesis is accompanied by a correspondence between (1) a decrease in the Hb concentration, resulting from a reduction in red blood cell production, and (2) an increase in plasma sTfR1, indicating an inadequate supply of iron for red blood cell production and tissue iron requirements.10-14 The concurrent increase in sTfR1 provides evidence that the decrease in Hb results from declining body iron stores, as indicated by SF concentrations and not by the many other conditions that can diminish red blood cell production. Accordingly, correspondence between the SF concentration at which the Hb concentration begins to decline and the sTfR1 concentration begins to rise provides a physiological basis for identifying an SF concentration that clinically identifies the onset of ID without anemia.7-9
The World Health Organization (WHO) recently determined that insufficient data are available to revise the current SF threshold for ID of <15 μg/L for the first trimester of pregnancy, which was based on expert opinion; WHO made no recommendations for the second and third trimesters.15 The overall certainty of the evidence for this guideline was determined as "low to very low," and establishing cutoff points by trimester was identified as a research gap. The WHO also noted that determining a ferritin concentration to define ID during pregnancy is complicated by "physiological changes occurring in hormones, blood composition, and hemodynamics, as well as in inflammatory status."15 In 1998, the Centers for Disease Control and Prevention (CDC) released recommendations to prevent and control ID in the United States. CDC used SF ≤15 μg/L as the threshold for defining ID in all individuals aged >6 months, based on a single small study of adult, nonpregnant women.16
In healthy pregnant women, we hypothesized that the clinical onset of ID without anemia could be identified in a similar manner by a correspondence between the SF concentration at which both decreases in Hb and increases in sTfR1 begin. During a normal iron-replete pregnancy, the average concentrations of the iron-regulatory hormone, hepcidin, are at prepregnancy levels during the first trimester but then fall to very low or undetectable levels during the second and third trimesters.17-20 In healthy, iron-replete pregnant women, accelerated erythropoiesis produces an increase of ∼35% in the circulating red blood cell mass, but the Hb concentration decreases as the result of an expansion in plasma volume of ∼50%.17,21 Plasma sTfR1 varies with erythropoietic and placental needs during the course of pregnancy.14 Given the array of hemodynamic, hormonal, and inflammatory alterations occurring during the course of pregnancy, we hypothesized that Hb and sTfR1 would vary by trimester in a similar manner among populations of healthy women with adequate iron stores. Compared with this uniform background of variation, the development of ID in a subpopulation of healthy pregnant women would simultaneously decrease the Hb and increase sTfR1 concentrations relative to those in women with iron-replete pregnancies.
Uncomplicated ID develops as the result of a dietary iron intake that is unable to meet iron requirements. During the second and third trimesters of pregnancy, the iron needs of the fetus and placenta increase beyond amounts that can be absorbed from the diet, and body iron stores are mobilized.21 Subsequently, 3 successive stages in the development of ID have been identified clinically as follows: (1) iron depletion, as body iron stores are reduced, but the iron supply for tissue requirements and erythropoiesis is maintained; (2) ID without anemia (nonanemic ID), as the synthesis of iron-dependent compounds and Hb are decreased; and (3) ID anemia (IDA), as the circulating Hb concentration falls beneath the standard used to define anemia, and production of iron-requiring compounds continues to decline.22-24 Clinically, the initial stage of ID, ID without anemia, is identified by a reduction in circulating Hb resulting from iron-restricted (iron-deficient) erythropoiesis. In otherwise healthy women who are not pregnant, ID, with or without anemia, is associated with fatigue, poor physical performance, and diminished work productivity.15 During pregnancy, the effects of ID on the evolution of the iron supply to the placenta and fetus, the erythroid marrow, and nonerythroid tissues are poorly understood. In pregnancy, ID without anemia is associated with an increased subsequent risk of anemia, placental hypertrophy, and thyroid dysfunction.25,26 ID with anemia is linked to preterm birth, placental abruption, preeclampsia, eclampsia, cesarean delivery, decreased quality of life, postpartum hemorrhage, hysterectomy, antenatal and postnatal maternal sepsis, need for blood transfusion, and, if severe, maternal death.25
In this study, we extend a physiologically based method of determining SF thresholds for ID by examining the relationship of SF with Hb and sTfR1 during each trimester of pregnancy in cross-sectional population data. No nationally representative data are available for healthy US pregnant women. Still, SF, Hb, and sTfR1 have been measured cross-sectionally in pregnant women in the US National Health and Nutrition Examination Survey (NHANES) since 1999. The continuous high-quality data from NHANES, using standardized laboratory analyses, in conjunction with the additional available information about participants’ health conditions, allows for data exclusion from individuals with other common causes of anemia at the population level that are independent of ID. After exclusions, NHANES data cannot be used to create nationally representative estimates of “healthy” US pregnant women. Therefore, in this study, we define and use the individuals in this subsample of NHANES data as an “apparently healthy sample.” Analysis of this apparently healthy sample allows for us to test the hypothesis that physiologically based SF concentration thresholds for ID during healthy pregnancies by trimester can be identified by examining the relationships between SF, Hb, and sTfR1 in cross-sectional data.
Methods
Study design and participants
NHANES is a multitopic survey designed to assess the health and nutritional status of adults and children in the United States. Currently, NHANES is a continuous survey (1999 to present) that includes a household interview followed by a standardized health examination in a mobile examination center.27 NHANES, conducted by the National Center for Health Statistics (NCHS), relies on a stratified multistage probability sample representative of the civilian, noninstitutionalized US population that is based on the selection of counties, blocks, households, and persons within households.27 Pregnant women are sampled based on the distribution of women of reproductive age. Ethical approval (https://www.cdc.gov/nchs/nhanes/irba98.htm) and procedures for assay data collection and analysis, as well as quality control and assurance, are described elsewhere.27-34 Ethics approval was obtained from the NCHS Ethics Review Board, and written informed consent was obtained from participants aged ≥12 years. Parental consent was obtained for those aged <18 years.
NHANES measured SF and sTfR1 during 1999 to 2010 and 2015 to 2018 but not during 2011 to 2014 due to budgetary constraints. For this analysis, we combined data from 1999 to 2010 and 2015 to 2018.27-34 We restricted our study sample to pregnant women aged 15 to 49 years who received health examinations in mobile examination centers (n = 1524). NHANES did not collect information on iron supplementation. We then identified an apparently healthy sample by using NHANES available data to exclude individuals with other common causes of anemia at the population level, independent of ID, namely, pregnant women with an indicator of inflammation or potential liver disease. Inflammation was measured by elevated C-reactive protein >5.0 mg/L,35 and potential liver disease was measured by abnormal alanine aminotransferase >70 U/L or aspartate aminotransferase >70 U/L.36 In addition, we excluded participants with SF concentration of >150 μg/L based on the WHO cutoff for severe risk of iron overload.15 The final sample for this analysis was 1288; details on sample selection are provided in Figure 1. The sample of 1288 pregnant women included 248 women with unknown trimester. Consequently, the sample size for the analyses by trimester was 1040. The features of laboratory analyses used, changes in methods from 1999 to 2018, and adjustments to data for methodological changes are provided as supplemental Material.
Flowchart of the selection of a healthy sample of pregnant women (15-49 years) participating in the NHANES 1999 to 2010 and 2015 to 2018. Pregnant women with an SF >150 μg/L were excluded from the healthy sample because of an increased risk of iron overload.15 Total and by each trimester (first, second, third, and unknown)
Flowchart of the selection of a healthy sample of pregnant women (15-49 years) participating in the NHANES 1999 to 2010 and 2015 to 2018. Pregnant women with an SF >150 μg/L were excluded from the healthy sample because of an increased risk of iron overload.15 Total and by each trimester (first, second, third, and unknown)
Statistical analysis
We first log-transformed SF (log10[ferritin]) data to normalize the distributions, calculated geometric means and 95% confidence intervals (CIs), and described the SF distributions by characteristics. We described the SF distributions (geometric means, 50th [median], 5th, and 95th percentiles) for survey cycle, age groups, trimesters, parities, racial/ethnic group, education, and family income. For the basic characteristics, SF distribution analysis and prevalence estimates, we used SUDAAN (version 11.0.1; Research Triangle Institute, Research Triangle Park, NC) with sample examination weights (2-year examination weights to calculate the overall weight across the years 1999 to 2010 and 2015 to 2018) and design variables to account for the complex sample design. We calculated both crude and adjusted geometric means for SF, and we used multiple linear regression models for the calculation of adjusted mean SF. A Bonferroni adjustment37 was used to correct the P values for the significance test for multiple comparisons across each sociodemographic characteristic in SF.
Using exploratory data analysis techniques,38 we examined monotonic relationships between Hb and SF and between sTfR1 and SF, using a scattergram superimposed with a plot of concentrations of median Hb and median sTfR1 according to 5 μg/L categories of SF levels by trimester. We used multivariate adjusted restricted cubic spline (RCS) regression models with 5 knots39-41 to examine the relationship of the following: (1) continuous SF with Hb; and (2) continuous SF with sTfR1 to identify the potential SF thresholds for ID. For each trimester, the multivariate RCS was adjusted by age in years and parity. Due to high percentages of missing parity information in the unknown trimester group and in the overall sample, the multivariate RCS were adjusted by age in years only. We used 2 complementary analyses to achieve our objectives. First, with Hb as our outcome variable, we modeled its relationship with SF and then solved for the SF concentration corresponding to the Hb plateau. Second, to further ascertain the consistency of this derived SF threshold, we modeled SF against sTfR1 and solved for the SF value corresponding to the sTfR1 minima. Using the fitted function of these 2 separate RCS analyses, we solved the ordinary differential equation derivative solutions at the plateau or minima of each model in SF units. The sample examination weights assigned to data were not included in the RCS analysis because it was not our objective to perform population-based analyses. All plateau and minima estimates and their 95% CIs were obtained from 5000 bootstrap replicates. All CIs have been corrected for bias using the bias corrected acceleration approach.42 Additionally, using the full NHANES SF and Hb data (no exclusions to create the healthy populations), we calculated the prevalence of ID and IDA using thresholds of the current WHO, current CDC, and new physiologically based thresholds from this analysis.
The NHANES ethical approval link is https://www.cdc.gov/nchs/nhanes/irba98.htm.
Results
Adjusted geometric mean SF concentrations were comparable through all NHANES cycles except 2003 to 2004, which was higher than the rest of the years 1999 to 2002, 2005 to 2010, and 2015 to 2018 (Table 1). The younger age group (15-24 years) had lower adjusted mean SF than pregnant women aged ≥25 years. First trimester pregnant women had higher adjusted mean SF than pregnant women in second and third trimesters. Furthermore, pregnant women with parity <2 had higher adjusted mean SF than women with a parity of ≥2 (Table 1). There was no statistical difference in adjusted mean SF among all racial/ethnical groups. There were no statistical differences in mean SF by education or family income groups (Table 1).
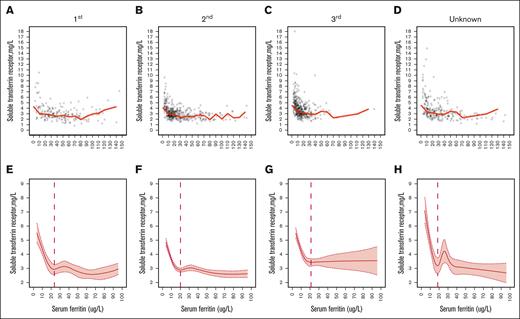
The median Hb-to-SF and sTfR1-to-SF plots by trimester are shown in Figure 2 (Hb) and Figure 3 (sTfR1). These empirical distribution plots indicated clear nonlinear trends with distinct thresholds conspicuous at certain levels of SF (x-axis) and inflection points at levels of Hb or sTfR1 (y-axis). Using RCS regression analysis, we found curvilinear relationships between continuous SF and Hb and between SF and sTfR1 for each trimester. In Hb, the SF values’ corresponding plateau points at first, second, and third trimesters were 25.8 μg/L, 18.3 μg/L, and 19.0 μg/L, respectively (Table 2; Figure 2). The identified threshold at first trimester (25.8 μg/L) was statistically higher than the thresholds at second (18.3 μg/L) and third trimesters (19.0 μg/L) but the thresholds between second and third trimesters were not significantly different statistically. Similar thresholds were also observed from the sTfR1-to-SF RCS analysis (Table 2; Figure 3).
Plot of SF (μg/L) concentrations with median Hb (g/dL) by trimester. First trimester (A), second trimester (B), third trimester (C), and unknown trimester (D); note that the behavior of the median becomes erratic with the small numbers of participants at the highest SF levels. Additionally, related RCS regression with 5 knots is shown. First trimester (E), second trimester (F), third trimester (G), and unknown trimester (H); vertical line indicates the plateau point; shaded areas inside the dashed lines are 95% CIs. Data from a healthy sample of pregnant women (aged 15-49 years) who participated in the NHANES from 1999 to 2010 and 2015 to 2018.
Plot of SF (μg/L) concentrations with median Hb (g/dL) by trimester. First trimester (A), second trimester (B), third trimester (C), and unknown trimester (D); note that the behavior of the median becomes erratic with the small numbers of participants at the highest SF levels. Additionally, related RCS regression with 5 knots is shown. First trimester (E), second trimester (F), third trimester (G), and unknown trimester (H); vertical line indicates the plateau point; shaded areas inside the dashed lines are 95% CIs. Data from a healthy sample of pregnant women (aged 15-49 years) who participated in the NHANES from 1999 to 2010 and 2015 to 2018.
Plot of SF (μg/L) concentrations with median sTfR1 (mg/L) by trimester. First trimester (A), second trimester (B), third trimester (C), and unknown trimester (D); note that the behavior of the median becomes erratic with the small numbers of participants at the highest SF levels). In addition, related RCS regression with 5 knots is shown. First trimester (E), second trimester (F), third trimester (G), and unknown trimester (H); vertical line indicates the plateau point; shaded areas inside the dashed lines are 95% CIs. Data from a healthy sample of pregnant women (15-49 years) who participated in the NHANES from 1999 to 2010 and 2015 to 2018.
Plot of SF (μg/L) concentrations with median sTfR1 (mg/L) by trimester. First trimester (A), second trimester (B), third trimester (C), and unknown trimester (D); note that the behavior of the median becomes erratic with the small numbers of participants at the highest SF levels). In addition, related RCS regression with 5 knots is shown. First trimester (E), second trimester (F), third trimester (G), and unknown trimester (H); vertical line indicates the plateau point; shaded areas inside the dashed lines are 95% CIs. Data from a healthy sample of pregnant women (15-49 years) who participated in the NHANES from 1999 to 2010 and 2015 to 2018.
Table 3 compares the differences between the proportions of pregnant women classified with ID or IDA using thresholds of the current WHO, current CDC, and new physiologically based thresholds from this analysis. The current WHO guideline offers SF threshold for only first trimester pregnant women, so only the first trimester prevalence of ID and IDA can be calculated. In general, physiologically based thresholds identified more ID without anemia than those using the current WHO threshold or current CDC threshold (Table 3).
Discussion
Our results identify physiologically based SF thresholds for ID in an apparently healthy sample of US pregnant women of ∼25 μg/L for the first trimester and ∼20 μg/L for the second and third trimesters. These thresholds use RCS analysis of Hb and sTfR1 concentrations (Table 2; Figures 2 and 3) in NHANES cross-sectional data. During each trimester, median Hb concentrations fall and median sTfR1 concentrations rise concurrently at the lowest serum SF concentrations that indicate diminished or absent body iron stores. At distinct thresholds at higher SF values, the median Hb and sTfR1 concentrations then plateau as SF rises with increasing body iron stores. To our knowledge, these are the first physiologically based trimester-specific SF thresholds to define ID during pregnancy that have been identified. Overall, these results indicate that an SF concentration threshold of 15 μg/L fails to identify ∼10% of US pregnant women with physiological evidence of ID (Table 3). These SF thresholds for ID in pregnancy in the first and second trimesters should not be extrapolated to judge iron sufficiency later in pregnancy. For comparison, without iron supplementation, one estimate is that a prepregnancy ferritin concentration of >50 μg/L is required to avoid the development of ID during pregnancy.43
The physiologically based SF thresholds from this study are higher than those from the current WHO (<15 μg/L, first trimester only) and the current CDC (≤15 μg/L) guidelines, which identify a more advanced stage of established ID. For the WHO guideline, ID was considered to exist when bone marrow iron staining is absent.14,15 This criterion confirms a diagnosis of established ID. Earlier studies of bone marrow iron in healthy nonpregnant women, which were used to estimate an SF threshold of 15 or 16 μg/L for absent iron stores, acknowledged that evidence of iron-deficient erythropoiesis developed at higher SF concentrations.44,45 Physiologically, ID is present when the iron circulating in the blood cannot provide the amounts required for red blood cell production, tissue needs, and, in pregnancy, fetal and placental needs.45,46 Our previous results in children and nonpregnant women and the results in this study of pregnant women add to the evidence that a small amount of storage iron, indicated by the higher SF thresholds from the NHANES data, may be present during the initial development of ID. Iron-deficient erythropoiesis begins before the absolute exhaustion of marrow iron stores.13,21,45-47 By identifying ID in women at an earlier stage of pregnancy, the physiologically based thresholds identify greater proportions as iron deficient during each trimester (Table 3). Recognizing the need for iron in more women during pregnancy could help prevent untreated ID, with the attendant risks of progressing to a greater iron deficit and resulting in anemia.
A recent review highlighted the need for gestational age–specific reference intervals for appropriate clinical decision-making.48 The physiologically based method estimates the mean SF threshold concentrations for ID during each trimester of pregnancy. After a clinically recognized pregnancy, the arrest of menstruation decreases iron losses to a basal level for much of the first trimester. Initially, a reduction in erythropoiesis may slightly reduce the circulating red blood cell mass and increase iron stores and hepcidin concentrations.18 In the latter portion of the first trimester, erythropoiesis starts to increase, fetal and placental growth accelerate, and plasma volume expansion begins. Over the entire first trimester, the mean SF does not change significantly from prepregnancy concentrations.18 The SF threshold for ID of about 25 μg/L is about the same as that for nonpregnant women of reproductive age.7 During the second trimester, as fetal and placental growth increase, hepcidin concentrations are greatly decreased, allowing iron stores to be mobilized to support the steady expansion in the red blood cell mass. Despite the increased red blood cell mass, the circulating Hb concentration falls because of the greater proportional increase in plasma volume. In the third trimester, fetal and placental growth, together with expansion of the red blood cell mass and plasma volume, continue rising to a peak in the latter portion of the trimester.21 Hepcidin concentrations remain extremely decreased, allowing remaining iron stores to be used to meet the steadily increasing iron needs. Mean SF concentrations decline during the second trimester and fall further during the third trimester.19 In contrast, SF threshold concentrations for ID of about 20 μg/L did not differ significantly during the second and third trimesters but both were lower than the threshold of about 25 μg/L during the first trimester.
These changes in the SF physiological thresholds for ID track those of hepcidin, the iron-regulatory hormone that controls the mobilization of iron stores.17 The molecular mechanism(s) underlying the suppression of hepcidin during the second and third trimesters have not been characterized but have been suggested to be of placental origin.49 Hepcidin was not measured during the NHANES surveys but the pattern of changes during pregnancy is well described.17-20,40 During the first trimester, the mean hepcidin concentration is little changed from prepregnancy levels, because early minor increases with the cessation of menstruation are offset by later decreases with the acceleration of erythropoiesis and of fetal and placental growth. During the second and third trimesters, mean hepcidin concentrations plummet to similar nadirs.19 The physiologically based SF thresholds for ID parallel the behavior of hepcidin concentrations, not differing from prepregnancy values during the first trimester and then declining to similar concentrations in the second and third trimesters. Comparable parallels are found between SF thresholds for ID and hepcidin levels because both increase when comparing children with nonpregnant women.7
Both epidemiological evaluation of iron status in pregnancy and clinical management are hindered at present by the substantial variation in SF thresholds to define ID.50 As noted above, the WHO 2020 guidelines (first trimester only)15 and the CDC 1998 guidelines16 recommend SF ≤15 μg/L as the threshold for ID in pregnancy. Other common thresholds for SF to define ID in studies of iron and micronutrient supplementation in pregnant women include SF <6, <10, <12, <15, <20, <30, <50, or <60 μg/L.50 This variability among guidelines is due, in part, to the reliance on expert opinion or on analysis of SF concentrations alone and contributes to a lack of consensus on recommendations for screening and management of ID in pregnancy. The use of a physiologically based method for establishing thresholds for ID in pregnancy could contribute to harmonization among guidelines. Still, before the adoption of physiologically based SF thresholds in pregnancy to guide clinical care and epidemiological evaluation, validation in non-US populations is needed, together with the exploration of the connection of these thresholds to functional consequences for the health of the mother and infant.51
Our study is subject to the limitations common to analysis of Hb and sTfR1 in cross-sectional data from NHANES that have been previously described, including identifying healthy individuals, changes in laboratory methods,7 and, during pregnancy, the lack of information about the use of iron supplements. Despite these constraints, our results provide evidence that current WHO (<15 μg/L, first trimester only) and current CDC (≤15 μg/L) guidelines may underestimate the prevalence of ID during pregnancy (Table 3) and fail to identify a substantial proportion of women who are iron deficient. The consequences for the health of the mother and child of failing to recognize and manage ID in these women during pregnancy have not been rigorously examined but, given the information already available, need to be determined.
Acknowledgment
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of Centers for Disease Control and Prevention.
Authorship
Contribution: Z.M., G.M.B., and O.Y.A. designed the analysis; Z.M. and O.Y.A. analyzed the data; Z.M. and G.M.B. wrote the manuscript; Z.M., O.Y.A., M.E.J., R.C.F.A., and G.M.B. interpreted the data and revised the manuscript; and all authors approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Zuguo Mei, Centers for Disease Control and Prevention, 4770 Buford Highway, MS S107-5, Atlanta, GA 30341-3724; email: zam0@cdc.gov.
References
Author notes
The US National Health and Nutrition Examination Survey (NHANES) data are in the public domain and can be downloaded from the NHANES website at https://www.cdc.gov/nchs/nhanes/.
The full-text version of this article contains a data supplement.