Key Points
CAR-Ts resulted stronger responses than BiAbs but lacked clinical significance with regards to PFS.
CAR-Ts followed by BiAbs could be 1 of the best treatment sequences for managing triple-class refractory myeloma.
Visual Abstract
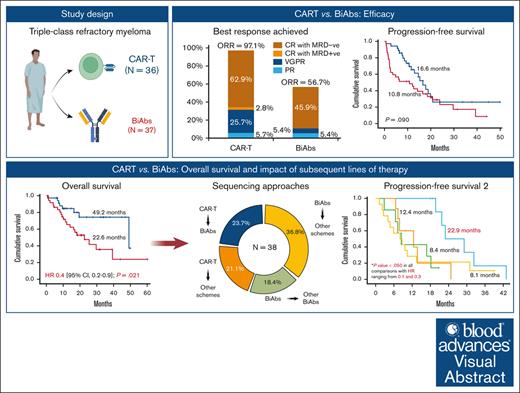
The efficacies of chimeric antigen receptor T cells (CAR-Ts) and bispecific monoclonal antibodies (BiAbs) for triple-class refractory (TCR) myeloma have not previously been compared, and clinical data on how to rescue patients after relapse from these immunotherapies are limited. A retrospective study of 73 TCR patients included in trials was conducted: 36 received CAR-Ts and 37 received BiAbs. CAR-Ts produced a higher overall response rate (ORR) than BiAbs (97.1% vs 56.8%, P = .002). After a median of follow-up of 18.7 months, no significant difference in progression-free survival (PFS) was observed between the CAR-T and BiAbs groups (16.6 vs 10.8 months; P = .090), whereas overall survival (OS) was significantly longer in the CAR-T than in the BiAbs group (49.2 vs 22.6 months; P = .021). BiAbs after CAR-Ts yielded a higher ORR and longer PFS2 than did nonredirecting T-cell therapies after CAR-Ts (ORR: 87.5% vs 50.0%; PFS2: 22.9 vs 12.4 months). By contrast, BiAbs after BiAbs resulted in an ORR of 33% and PFS2 of 8.4 months, which was similar to that produced by the nonredirecting T-cell therapies (ORR: 28.6%; PFS2: 8.1 months). Although this is a pooled analysis of different trials with different products and the patient profile is different for CAR-Ts and BiAbs, both were effective therapies for TCR myeloma. However, in our experience, although the PFS was similar with the 2 approaches, CAR-T therapy resulted in better OS, mainly because of the efficacy of BiAbs as rescue therapy. Our results highlight the importance of treatment sequence in real-word experience.
Introduction
Multiple myeloma (MM) is the second most common hematological malignancy, and the introduction and combination of proteasome inhibitors (PIs), immunomodulators (IMiDs), and anti-CD38 monoclonal antibodies (moAbs) has yielded response, progression-free survival (PFS), and overall survival (OS) rates previously inconceivable in patients with MM.1,2
Nevertheless, MM remains an incurable disease in the majority of patients, because relapses are common and patients are very likely to receive different drug combinations throughout the course of their disease.3,4 Therefore, offering optimal treatment to patients already exposed to PIs, IMiDs, and anti-CD38 moAbs (namely triple-class exposed [TCE]) and/or refractory to these drugs (triple-class refractory [TCR]), represents a therapeutic challenge. The LocoMMotion and MAMMOTH studies have highlighted the dismal outcomes of patients with TCE/TCR myeloma, showing suboptimal responses (∼30%) and complete response (CR) or better (≥CR) of <1%. Indeed, the PFS and OS of this subset of patients were not longer than 6 months and 1 year, respectively.5,6
The medical need was met by developing novel therapeutic approaches such as T-cell–redirecting therapy, including bispecific monoclonal antibodies (BiAbs) and chimeric antigen receptor T-cells (CAR-Ts) targeting B-cell maturation antigen (BCMA), with overall response rates (ORRs) of 70% to 100%, ≥CRs of 30% to 60%, and median PFSs of 9 to 36 months in TCE/TCR patients.7-9 Furthermore, BiAbs directed against other targets, such as G protein–coupled receptor class C group 5 (GPRC5D) and Fc receptor homologs 5, are effective in treating TCE/TCR myeloma, giving ORRs of 50% to 70% and median PFSs of ∼1 year.10-16 However, no studies have compared the outcomes with both therapies.
In addition, relapse/refractory MM (RRMM) after CAR-Ts or BiAbs has become another challenging scenario because clinical data are limited and effective treatment options are lacking in this population.17,18 In this regard, it would be important to address the optimal treatment sequence.
We aim to conduct a retrospective study to compare the efficacy of CAR-Ts and BiAbs strategies, and the prognosis of patients who have relapsed after both therapies and the best treatment sequence, as well as the logistics and safety profile of patients with TCR myeloma treated with both therapies.
Methods
A retrospective observational study was designed including patients with MM enrolled in clinical trials and treated with CAR-Ts and BiAbs at the University Hospital of Salamanca between December 2018 and May 2023. The follow-up cutoff date was 30 November 2023. This study was approved by the institutional review boards of our institution and conducted in compliance with the Declaration of Helsinki.
Only patients with TCR myeloma were included to mitigate clinical and statistical biases, and their characteristics were assessed at relapse before initiating treatment. Fluorescence in situ hybridization studies were carried out in separated plasma cells, as previously reported19; and t(4;14), t(14;16), and 17p deletion [del(17p)] were designated as high-risk cytogenetic abnormalities (HRCA), with a cutoff level of 10%. Extramedullary disease (EMD) was defined as the presence of plasmacytomas without bone contact and was identified by positron emission tomography/computed tomography.
Responses were assessed according to the 2016 International Myeloma Working Group criteria.20 ORR was defined as a partial response (PR) or better response (≥PR). Early assessment was that performed in the first month after starting both treatments. A bone marrow aspiration was performed, according to the 2016 International Myeloma Working Group guidelines, to confirm suspected CR and to measure minimal residual disease (MRD), as previously described.21
PFS was defined as the time from CAR-T infusion or the first dose of BiAbs (T0) to disease progression or death from any cause, whichever occurred first; PFS 2 (PFS2) was defined as the time from T0 to disease progression or death from any cause after the next line of treatment, whichever occurred first; OS was defined as the time from T0 to death from any cause.
Time to treatment access was defined differently depending on the type of immunotherapy received. In the CAR-T group, this was the vein-to-vein time, that is, the time between leukapheresis and T0. In the BiAbs group, time to access was defined as the time between the date of the screening and T0.
Hematological adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events 5.0. Immune-related adverse events were graded according to the American Society for Transplantation and Cellular Therapy criteria.22,23
Differences between the CAR-T and BiAbs groups were estimated using the χ2 and Mann-Whitney U tests for qualitative and quantitative variables, respectively. χ2 tests identified statistically significant differences between categories of qualitative variables; the associated odds ratio (OR) and 95% confidence interval (95% CI) were estimated by logistic regression. The distributions of PFS and OS were estimated using the Kaplan-Meier method, the differences were examined using the log-rank test, and the corresponding hazard ratio (HR) and 95% CI were estimated by Cox regression. Values of P < .05 were considered significant. Statistical analyses were performed with IBM SPSS Statistics, version 28.
This study was approved by the institutional review boards of University Hospital of Salamanca.
Results
In total, 73 TCR patients were treated with T-cell–redirecting therapy at our institution during the aforementioned period; 36 patients were treated with CAR-Ts, and 37 patients received BiAbs. In all patients treated with CAR-Ts, the target was BCMA. Within the BiAbs group, 21 patients received BiAbs targeting BCMA, 10 received BiAbs targeting GPRC5D, 3 received the combination of BiAbs targeting BCMA and GPRC5D, and 3 received BiAbs targeting Fc receptor homologs 5. The different products received by both groups are detailed in supplemental Table 1. The median time to receiving T-cell–redirecting therapy after diagnosis was 63.7 months (range, 7.6-286.5 months). No significant differences in this time were observed between the CAR-T and BiAbs groups (63.3 vs 65.3 months; P = .557).
Clinical characteristics and prior lines of therapies of TCR patients
Overall, patients had a median age of 62 years (range, 36-82 years); 60.3% of patients were men. Approximately one-third presented EMD, one-third harbored HRCA, and 1 in 5 patients had International Staging System stage 3 disease (Table 1). Regarding previous treatments, the median number of prior lines of therapy (LOT) was 3 (range, 2-7 LOT), 61.6% were penta-exposed, 23.3% were penta-refractory, and 82.2% had received at least 1 autologous stem cell transplantation. In addition, few patients had been previously treated with the BCMA antibody drug conjugate belantamab mafodotin (n = 4; 5.5%) or allogeneic stem cell transplantation (n = 2; 2.7%).
Patients from CAR-T group were younger than those who received BiAbs (≥70 years: 11.1% vs 35.1%; P = .015) and had lower EMD (19.4% vs 45.9%; P = .009), no other significant differences were observed in baseline characteristics. The 2 groups were well balanced in terms of prior exposure and refractoriness to PIs, IMiDs, and anti-CD38 moAbs (Table 1). However, compared with the BiAbs group, patients in the CAR-T group were less frequently pretreated, with a median prior LOT of 3 (range, 2-6 LOT) vs 4 (range, 2-7 LOT; P = .003), and 30.5% of patients receiving ≥4 prior LOT vs 67.6% (P = .002).
Response
Overall, 72 patients were evaluable. At the early assessment, ORR was achieved in 53 patients (73.6%), with 10 patients (13.9%) in CR. Best response was ≥PR in 55 patients (76.4%) and ≥CR in 40 patients (55.6%), with MRD negativity in 39 patients (54.2%).
At the early assessment (supplemental Figure 1), the ORR of CAR-T group was significantly higher than in the BiAbs group: 94.3% vs 54.1% (OR, 14.0; 95% CI, 2.9-67.2; P < .001). Notably, 22.9% of patients receiving CAR-T infusion reached ≥CR, significantly more than the 5.4% of patients treated with BiAbs (OR, 5.2; 95% CI, 1.1-26.4; P = .048). Indeed, MRD negativity was more frequently achieved in the CAR-T than in the BiAbs group (20.0% vs 2.7%; OR, 9.0; 95% CI, 1.1-77.5; P = .045).
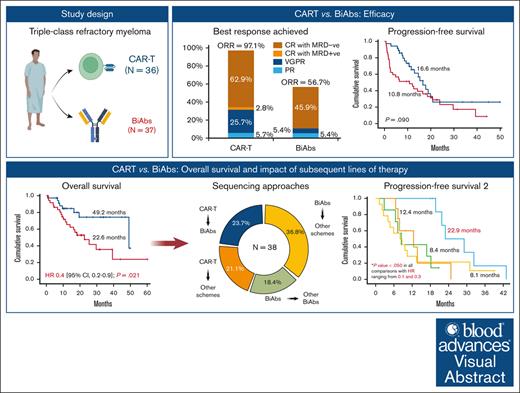
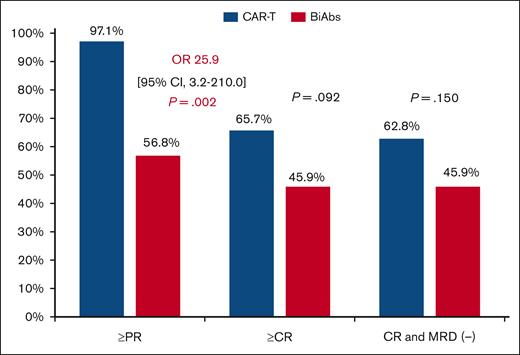
Significant differences in ORR were maintained during follow-up (Figure 1), with 97.1% in the CAR-T and 56.8% in the BiAbs group (OR, 25.9; 95% CI, 3.2-210.0; P = .002). Both groups showed improved responses as the treatment proceeded, with 65.7% and 45.9% of patients treated with CAR-T and BiAbs, respectively, upgrading their response (P = .092). MRD-negative cases were more frequent, although not significantly so, in the CAR-T group than in the BiAbs group: 62.8% vs 45.9% (P = .150).
Best response achieved in TCR patients treated with T-cell–directing therapies.
Best response achieved in TCR patients treated with T-cell–directing therapies.
Survival
On the cutoff date, the median of follow-up was 18.7 months (range, 2.3-60.1 months) and the median PFS of the entire cohort was 14.3 months (95% CI, 17.3-24.0).
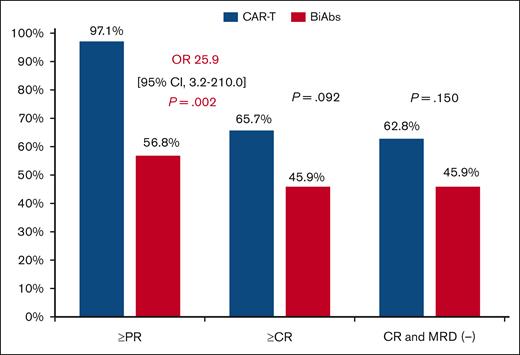
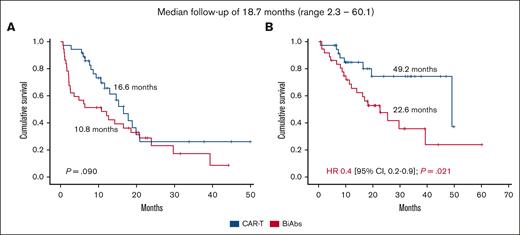
The median PFS of patients treated with CAR-Ts was 16.6 months, longer than the 10.8 months of those patients treated with BiAbs, but not statistically significant (P = .090; Figure 2A). The presence of EMD identified patients with poorer PFS compared with those without this feature (17.8 vs 6.0 months; HR, 2.9; 95% CI, 1.6-5.4; P < .001), whereas no differences were observed in patients with or without HRCA (17.8 vs 8.6 months; P = .318). No significant differences were observed in PFS between the CAR-T and the BiAbs group in patients displaying EMD (13.0 vs 2.2 months; P = .358) or HRCA (10.6 vs 4.8 months; P = .102; supplemental Figure 2). In contrast, patients who achieved CR with MRD negativity had longer PFS than those who did not in the CAR-T group (18.9 vs 10.6 months; HR, 0.3; 95% CI, 0.1-0.8; P = .014), and in the BiAbs group (23.9 vs 2.2 months; HR, <0.1; 95% CI, 0.0-0.1; P < .001; supplemental Figure 3). No significant differences were observed in patients who achieved CR with MRD negativity in both groups (supplemental Figure 4). It should be noted that, regardless of T-cell–redirecting therapy used, achieving CR with MRD negativity made it possible to improve the dismal prognosis associated with HRCA (CAR-Ts: standard-risk cytogenetic abnormalities vs HRCA: 19.9 vs 15.3 months, P = .516; BiAbs: standard-risk cytogenetic abnormalities vs HRCA: 29.7 vs 16.5 months; P = .153; supplemental Figure 5), whereas it did not do so at all in those with EMD (CAR-Ts: no EMD vs EMD: 18.9 vs 13.0 months, P = .198; BiAbs: no EMD vs EMD: 29.7 vs 16.5 months; HR, 0.1; 95% CI, 0.0-0.9, P = .038; supplemental Figure 6).
Survival of TCR patients treated with T-cell–directing therapies. (A) PFS and (B) OS.
Survival of TCR patients treated with T-cell–directing therapies. (A) PFS and (B) OS.
Considering the OS, 8 and 20 events occurred in the CAR-T and BiAbs groups, respectively. The main cause of death was progressive disease in both groups. The median OS in the entire cohort was 39.3 months (95% CI, 22.5-56.2). Significant differences were observed between patients treated with CAR-Ts and BiAbs: 49.2 vs 22.6 months (HR, 0.4; 95% CI, 0.2-0.9 months; P = .021; Figure 2B).
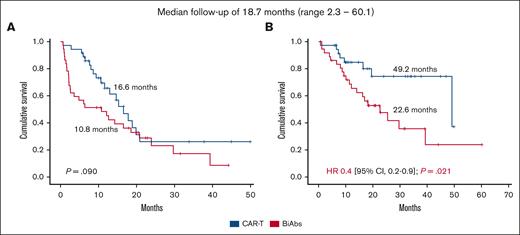
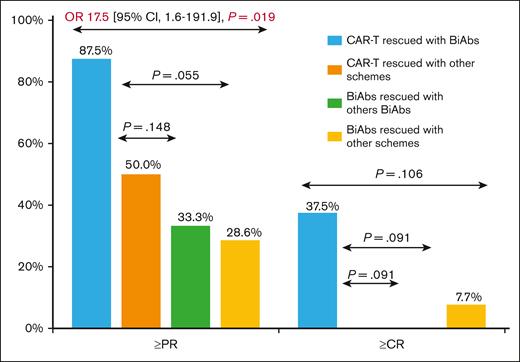
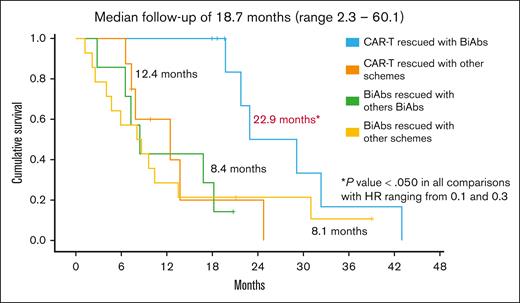
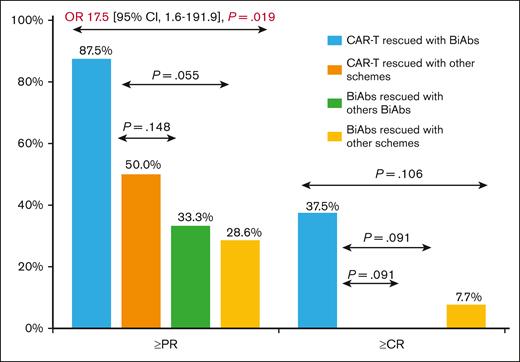
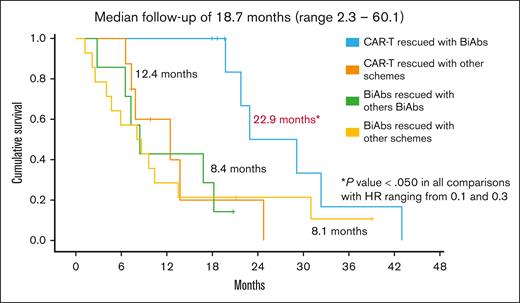
To establish the reasons for these differences in OS, we analyzed the impact of therapies administrated after CAR-Ts and BiAbs. Thirty-eight patients, 17 in the CAR-T group and 21 in the BiAbs group, were treated with salvage treatments after these novel immunotherapies, and almost half of them within clinical trials, with no differences between the 2 groups: 52.9% in the CAR-T group (n = 9) vs 33.3% in the BiAbs group (n = 7; P = .224; Table 2). In both groups, all but 3 patients rescued with T-cell–redirecting therapies changed the target (to GPRC5D in the majority of cases), the exceptions were 1 patient in the CAR-T group, who was treated again with BCMA CAR-Ts; and 2 patients, 1 each in the CAR-T and BiAb groups, who received the combination of anti-BCMA and anti-GPRC5D BiAbs. The median number of lines of salvage therapies was 1 (range, 1-4), and the ORR and ≥CR were 47.1% and 11.8%, respectively. To identify the optimal sequence, patients were stratified into 4 groups: CAR-Ts followed by BiAbs, CAR-Ts followed by other schemes, BiAbs followed by other BiAbs, and BiAbs followed by other schemes. Almost 90% of patients relapsing from CAR-Ts rescued with BiAbs yielded ≥PR and 38% ≥CR (Figure 3). The ORR was lower in the other 3 groups (30%-50%), with few deep responses. The median PFS2 was 13.5 months (95% CI, 5.4-21.6). The treatment sequence of CAR-Ts followed by BiAbs produced the longer PFS2 (Figure 4), median PFS2 22.9 months, as compared with CAR-Ts followed with other schemes (12.4 months; HR, 0.2; 95% CI, 0.1-0.7; P = .014), or BiAbs followed by BiAbs (8.4 months; HR, 0.1; 95% CI, 0.0-0.5, P = .011); or other schemes (8.1 months; HR 0.3; 95% CI, 0.1-0.9; P = .033).
Best response achieved in TCR patients rescued after T-cell–directing therapy failure.
Best response achieved in TCR patients rescued after T-cell–directing therapy failure.
PFS2 in TCR patients rescued after failure of T-cell–directing therapies.
Logistics: time to treatment access and duration of hospitalization
The median time to treatment access of the cohort was 34 days (range, 5-134 days). It is important to note that the time to treatment access differed dramatically, being significantly longer in the CAR-T group than in the BiAbs group: 56.5 days (range, 40-134 days) vs 14 days (range, 5-34 days; P < .001; supplemental Figure 7).
The median hospitalization time in our cohort was 19 days (range, 1-45 days). An important consideration is that patients who received CAR-Ts were hospitalized for twice as long as those receiving BiAbs, 24 days (range, 15-45 days) vs 12 days (range, 1-39 days; P < .001; supplemental Figure 8).
Safety profile
Toxicities with CAR-Ts and BiAbs are summarized in Table 3. Cytokine release syndrome occurred in 83.6% of patients treated with these novel immunotherapies and was slightly, but not significantly, more common in the CAR-T group (91.7%) than in the BiAbs group (75.7%; P = .065). In addition, 6.6% of patients in the entire cohort developed immune effector cell–associated neurotoxicity syndrome, with no significant differences between groups (5.5% in the CAR-T group vs 8.1% in the BiAbs group; P = .666). Of note, immune effector cell–associated hemophagocytic lymphohistiocytosis-like syndrome (IEC-HS) was an exclusive complication of those patients receiving CAR-T infusion (19.4% vs 0.0%; OR, not estimable; P = .005). Only 1 patient died, from grade 5 IEC-HS, but no other immune-related adverse events of grade ≥3 occurred.
Considering cytopenias, grade 4 neutropenia was present in 57.5% of patients in the cohort. Grade 4 neutropenia was much more frequent in the CAR-T group than in the BiAbs group: 97.2% vs 18.9% (OR, 150.0; 95% IC, 17.5-1289.4; P < .001). Likewise, patients treated with CAR-Ts more frequently presented grade ≥3 neutropenia than did patients treated with BiAbs at early- (<1 month; 94.4% vs 29.7; OR, 40.2; 95% CI, 8.2-197.2; P < .001) and midterm follow-up (1-3 months; 88.6% vs 38.7; OR, 12.3; 95% CI, 3.5-43.6; P < .001). By contrast, 42.5% of the cohort presented grade 4 thrombocytopenia, occurring more frequently in patients treated with CAR-Ts than in those treated with BiAbs (72.2% vs 13.5%; OR, 16.6; 95% CI, 5.1-54.8; P < .001). In fact, grade ≥3 thrombocytopenia was more common in the CAR-T group than in the BiAbs group at early- (72.2% vs 16.2%; OR, 13.4; 95% CI, 4.3-41.9; P < .001) and midterm follow-up (65.7% vs 9.7%; OR, 17.9; 95% CI, 4.5-71.1; P < .001). However, at long-term follow-up (>3 months), no statistically significant differences in grade ≥3 neutropenia or thrombocytopenia were observed.
The development of tumor lysis syndrome was anecdotal in our series, occurring in only 2 patients of the entire cohort. In contrast, hypogammaglobulinemia occurred in almost 3 out of 4 patients. Moreover, patients treated with CAR-Ts were 3 times more likely to develop hypogammaglobulinemia than patients in the BiAbs group (85.7% vs 64.9%; OR, 3.2; 95% CI, 1.1-10.4; P = .047). In the BiAbs group, hypogammaglobulinemia was comparable in patients treated with BiAbs directed against BCMA (62.5%) and with other targets (69.2%; P = .682).
Infections were balanced with both therapies and ∼70% of the patients in the entire cohort experienced at least 1 episode during follow-up. Infections increased progressively over time, and there was a trend toward fewer infections in the CAR-T group, especially at the mid- and long-term follow-ups (Table 3). At early follow-up, no clear patterns of infection were observed, whereas mild viral upper–respiratory tract infections were most frequently reported at mid- and late-term follow-ups. Additionally, 1 in 4 patients developed severe infections, the proportions being similar in both groups. Grade 5 infection was the cause of death in 3 patients in the BiAbs group (2 from severe acute respiratory syndrome coronavirus 2 pneumonia, and 1 from bacterial pneumonia).
Discussion
To the best of our knowledge, this is the first study that compares the efficacy, logistics, and safety profiles of CAR-Ts and BiAb therapies. This retrospective study demonstrated that both T-cell–redirecting therapies were effective in TCR patients. Notably, CAR-Ts resulted in better responses than BiAbs, although no significant benefit to PFS was observed. However, TCR patients treated with CAR-Ts presented significantly longer OS than those treated with BiAbs (49.2 vs 22.6 months; HR, 0.4; 95% CI, 0.2-0.9; P = .021). These differences in OS highlight the importance of stablishing a treatment plan. The sequence of CAR-Ts followed by BiAbs achieved better responses (ORR: 87.5%, and ≥CR: 37.5%) and significantly longer PFS2 (22.9 months) than other sequencing approaches, suggesting that BiAbs could be an optimal therapeutic option after CAR-Ts.
Overall, T-cell–directing therapies resulted in ≥PR in 3 of 4, and ≥CR in half of TCR patients. Moreover, T-cell–redirecting therapy allowed to achieve rapid responses that improve over time, with more than half of our patients becoming MRD negative. As expected, the achievement of MRD negativity resulted in longer PFS compared with PFS in patients who did not attain this response, regardless of the T-cell–directing therapies used. Zabaleta et al reported similar findings in a study of ∼250 patients treated with CAR-Ts and BiAbs.24 Therefore, T-cell–directing therapies yielded better outcomes for TCR myeloma than those reported in the LocoMMotion and MAMOTH studies,5,6 covering a medical need for these patients.
In our series, the response was significantly better and the PFS was longer (although not significantly so) in the CAR-T group than in the BiAbs group. These results are consistent with a recently published meta-analysis covering >2000 patients treated with these novel immunotherapies.25 However, it should be noted that patients of the BiAbs group of our series were older, more frequently pretreated, and presenting more EMD. Although patients received different CAR-T products based on our clinical trials availability, the median PFS observed in our series is not very different to that reported with idecabtagene vicleucel in the pivotal KarMMa trial (∼9 months)7 as well as with ARI-0002h in the CARTBCMAHCB-01 study (∼15 months).9 Ciltacabtagene autoleucel is the BCMA CAR-T product evaluated in the CARTITUDE-1 resulting in a median PFS of 35 months26 but it was not available in Spain. In contrast, the efficacy of BiAbs in our series was consistent with those reported in clinical trials of BiAbs.10-16 Of note, our results are likely to be better than those observed in the real world with CAR-T and BiAbs,27,28 because all patients were treated in clinical trials that have inherent selection bias.
HRCA and EMD are historically unmet medical needs of myeloma, often underrepresented in clinical trials, making it difficult to extrapolate results. In the TCE/TCR setting, the MAMMOTH trial and a pooled analysis of LocoMMotion and MoMMent revealed an OS of no longer than 6 months in patients with these high-risk features.6,29 In addition, CAR-Ts and BiAbs were less efficacious in both subsets of patients.25,30 Nevertheless, in our study, patients of the CAR-T group with EMD and patients of both groups with HRCA who attained MRD negativity had similar survival to that of patients without these features. Therefore, the design of clinical trials in these challenging populations with T-cell–redirecting therapies, even in a complementary way, with the goal of achieving MRD negativity, sustained over time, could improve or overcome the prognosis of these subset of patients.
One of the most relevant findings in our study was the significant differences in OS between patients treated with CAR-Ts and those treated with BiAbs. This clinical benefit favoring the CAR-T group highlights the feasibility and the efficacy of BiAbs after CAR-T relapse, illustrating that BiAbs targeting alternative antigens are probably an optimal rescue therapy. Similar findings were reported in a retrospective study in which T-cell–directing therapy used as a salvage therapy after CAR-Ts resulted in longer median OS compared with patients rescued with other approaches, including transplant-based approaches.18 In addition, this treatment sequence could be 1 of the most optimal because emerging data show how BiAbs retain their efficacy after CAR-Ts. This has been evidenced in the cohort C of the MajesTEC-1 in which teclistamab was effective in TCR patients previously exposed to BCMA CAR-Ts, with an ORR of ∼50% and duration of response of almost 1 years in 70% of responders.31 Indeed, the benefit of this sequence appears to be more evident when the target is switched to GPCR5D, and talquetamab in the phase 2 MonumenTAL-1 trial showed a >70% of ≥PR in those patients who relapsed after BCMA CAR-T.32 Contrary to our results, other authors have suggested that BiAbs could be a useful treatment in patients who relapsed after other BiAbs.17 However, recent data of clinical trials showed that the efficacy of BiAbs is impaired when treating a patient who has immediately relapsed after other BiAbs. In the MonumenTAL-1 study, the ORR of talquetamab was lower in patients previously treated with BCMA BiAbs (∼50%) than in patients who relapsed after BCMA CAR-Ts (∼70%).32 Also, in this trial, the sequence talquetamab followed by BCMA BiAbs produced worse responses than those rescued with BCMA CAR-Ts (33.3% vs 66%).33 In particular, patients rescued with CAR-Ts after BiAbs are not represented in our study; because BCMA was the primary target in the BiAbs group, prior exposure to BCMA was an exclusion criterion and most clinical trials evaluating CAR-Ts and CAR-Ts directed against GPRC5D was not available Spain. Nevertheless, different studies pointed out that previous exposure to BiAbs negatively affects the efficacy of CAR-T therapy. In this sense, a real-world study and the cohort C of CARTITUDE-2 have revealed how the efficacy of idecabtagene vicleucel and ciltacabtagene autoleucel was substantially decreased in patients previously exposed to BCMA BiAbs compared with the outcomes reported in the KarMMa (2.3 vs 8.8 months) and CARTITUDE-1 (5.3 vs 34.9 months) trials, respectively.34,35 Data on CAR-Ts after GPRC5D BiAbs are immature to draw conclusion, although they show promising response rate.33 Further studies in larger populations will help better understand the sequencing of therapies. In this regard, BCMA and GPRC5D mutations and their expression or T-lymphocyte functionality studies could be useful for choosing the most appropriate therapeutic sequence.36-38
Because CAR-Ts and BiAbs are effective in the TCR population, the choice of therapy needs to be properly evaluated. Time to therapy access is critically important because some patients experience an aggressive relapse requiring rapid treatment. Notably, vein-to-vein time in our CAR-T group (median, ∼2 months) was 4 times longer than access time to BiAbs, the latter being even shorter in the real-world setting. As expected, the CAR-T group presented more toxicity than the BiAbs group, more frequently developing cytokine release syndrome, IEC-HS, severe cytopenias, and hypogammaglobulinemia. Both groups were manageable, no new safety signals were reported, and our safety profile was slightly better than those reported in the aforementioned pivotal trials, except for IEC-HS. Interestingly, patients of the CAR-T group did not experience more infections despite their higher rates of neutropenia and hypogammaglobulinemia. Nevertheless, many patients in the BiAbs group were treated during the COVID-19 pandemic, which could have influenced the results. Therefore, safety profile and logistics data should be considered in clinical decision-making, especially in patients with very aggressive disease and comorbidities.
The sample size, and the retrospective and single-center nature are the most notable limitations of the study. As previously mentioned, patient selection bias is present in this study because most patients have been referred from other centers for being included in the trials. This fact does preclude the analysis based on an intention-to-treat population. Patients of both groups received different products, and those rescued with CAR-Ts are not represented in the study. Also, few patients presented HRCA and EMD, and only half the cohort was evaluated in the PFS2 analysis, which prevents more accurate conclusions being drawn, and therefore, subanalyses should be interpreted with caution. However, our study provides unique clinical data that may be helpful in patient selection and a guide to the possible standard of care in RRMM after these novel immunotherapies.
In conclusion, CAR-Ts and BiAbs have been shown to be effective in treating TCR myeloma. Responses and depth of responses, with high MRD negativity rates, even in high-risk patients, improved PFS in TCR patients. Therefore, the toxicity and logistics of both therapies should be considered to make the most appropriate clinical decisions. There is no standard of care for patients relapsing after CAR-Ts and BiAbs. The longer OS of patients in the CAR-T group demonstrates the importance of choosing an appropriate treatment sequence and that BiAbs are a feasible and effective treatment after CAR-T. The identification of therapeutic sequencing approaches and the development of new drugs creates a new unmet medical need in patients with RRMM after novel immunotherapy.
Acknowledgments
The authors thank the entire clinical trials and advanced therapies team for their support and work, Philip Mason for the language revision of the manuscript, and the “Fundación para el Desarrollo de la Hematología y Hemoterapia de Salamanca” for financial support.
Authorship
Contribution: B.P., A.F.-S., V.G.-C. and M.-V.M. conceived the study idea; B.P., E.A. and A.F.-S. provided the study materials; B.P., A.F.-S., V.G.-C., and M.-V.M. had full access to all the study data; B.P., V.G.-C., and M.-V.M. analyzed and interpreted the data, and wrote the original draft of the manuscript; B.R.-B., A.A.M.-L., E.P.-L., L.L.-C., N.P., V.G.-C., and M.-V.M. contributed to patient selection for both therapies; M.L.-P. coordinated the leukapheresis of patients treated with CAR-T; N.C.G.-G., R.G.-S., and N.P. coordinated the biological studies of the patients; and all the authors treated the patients, and reviewed, edited and approved the final version of the manuscript.
Conflict-of-interest disclosure: B.P. has received honoraria from Janssen, Amgen, and Aptitude Health. E.A. has received honoraria from Janssen. B.R.-B. has received a speaker’s fees from Janssen. L.L.-C. has received honoraria for lectures and/or advisory board activities from Kite-Gilead, Merck Sharp & Dohme, and Novartis. N.C.G.-G. has received honoraria from Janssen and Amgen. R.G.-S. has received honoraria from Janssen, Takeda, and Amgen; and has received research funding from Gilead Sciences and Incyte. N.P. has received honoraria for consulting or advisory roles from Amgen, Celgene, Janssen, Takeda, The Binding Site, GlaxoSmithKline, and Sanofi. V.G.-C. has received honoraria from Janssen and Celgene; reports research funding from Janssen; and received honoraria for consulting and advisory roles for Prothena and Janssen. M.-V.M. has received honoraria derived from lectures and advisory board activities from Janssen, Bristol Myers Squibb/Celgene, Amgen, Takeda, AbbVie, Sanofi, Oncopeptides, Adaptive, Roche, Pfizer, Regeneron, GlaxoSmithKline, Bluebird Bio, and Sea-Gen. The remaining authors declare no competing financial interests.
Correspondence: María-Victoria Mateos, Servicio de Hematología, Unidad de Ensayos Clínicos, Hospital Universitario de Salamanca, Paseo de la Transición S/N, 37007 Salamanca, Spain; email: mvmateos@usal.es.
References
Author notes
The data that support the findings of this study are available, upon reasonable request, from the corresponding author, María-Victoria Mateos (mvmateos@usal.es) and the first author, Borja Puertas (borjapuertas@usal.es).
The full-text version of this article contains a data supplement.