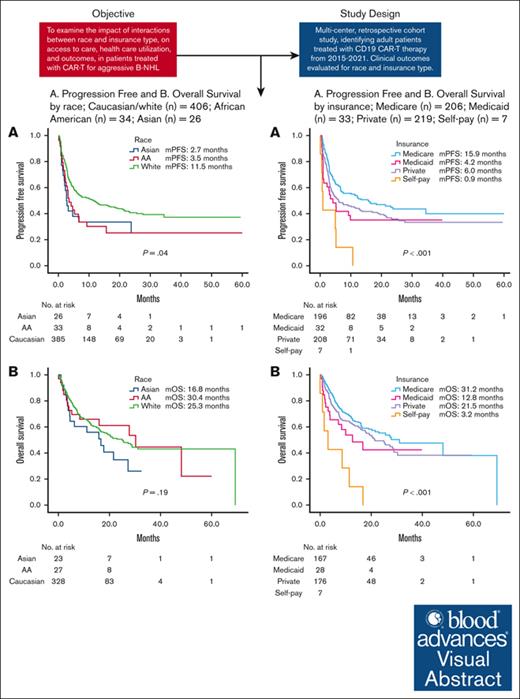
Racial disparities exist in patients who received CAR-T therapy for aggressive B-NHL.
Payor status affects survival, with improved survival noted in Medicare patients receiving CAR-T therapy.
Visual Abstract
Chimeric antigen receptor (CAR) T-cell (CAR-T) immunotherapy is an effective therapy for relapsed/refractory B-cell non-Hodgkin lymphoma (r/r B-NHL). However, data are limited on the impact of the convergence of race and social determinants of health on outcomes for patients treated with CAR-T therapy. We examined the impact of interactions between race and insurance type on health care use and outcomes in patients treated with CAR-T therapy for aggressive B-NHL. Adult patients with r/r B-NHL treated with CD19 CAR-Ts were identified between 2015 and 2021 across 13 US academic centers. Insurance type, demographic, and clinical data were collected and analyzed. In total, 466 adult patients were included in our analysis. Median follow-up after CAR-T therapy was 12.7 months. Median progression-free survival (mPFS) was longer for Caucasians (11.5 months) than for African Americans (3.5 months; hazard ratio [HR], 1.56 [1.03-2.4]; P = .04) or Asians (2.7 months; HR, 1.7 [1.02-2.67]; P = .04). Differences in median overall survival (mOS) were not significant. For Medicare (n = 206) vs Medicaid (n = 33) vs private insurance (n = 219) vs self-pay (n = 7): mPFS was 15.9 vs 4.2 vs 6.0 vs 0.9 months (P < .001), respectively; and mOS was 31.2 vs 12.8 vs 21.5 vs 3.2 months (P < .001), respectively. Our multicenter retrospective analysis showed that race and insurance status can affect outcomes for patients treated with CAR-T therapy.
Introduction
Health care disparities driven by multiple social, economic, and/or environmental factors lead to inequalities in health outcomes.1,2 Racial and ethnic disparities are further exacerbated by lower enrollment in clinical trials for new and promising therapies, which may ultimately lead to differences in outcomes and less use once these products are fully available on the market.3,4 Insurance status is often a factor that further compounds these disparities.5 Chimeric antigen receptor T-cell (CAR-T) therapy is a novel therapy for relapsed and refractory (r/r) B-cell non-Hodgkin lymphoma (B-NHL).6-8 CAR-T therapy is now recommended as third-line therapy and beyond for patients with diffuse large B-cell lymphoma as well as for second-line therapy for those who are transplant ineligible or who have primary refractory or relapsed disease occurring <12 months after first-line chemotherapy, a population in which outcomes have been traditionally very poor. Trials have shown that in the second-line setting, CAR-T therapy has improved progression-free survival (PFS) and overall survival (OS) as compared with autologous stem cell transplantation (SCT).9 However, there is evidence that past and present clinical trials for CAR-T therapy have had suboptimal accrual of patients from racial and ethnic minorities.10,11 For example, only 5% of patients on the pivotal Zuma 7 trial were Hispanic and 5% were African American (AA).12 As a result, data that examine the impact of race and social determinants of health on outcomes in patients treated with CAR-T therapy are limited. To address this gap, our objective was to examine the impact of interactions between race and insurance type, on access to care, health care use, and outcomes, in patients treated with CAR-Ts for aggressive B-NHL.
Methods
We conducted a multicenter retrospective cohort study that included patients from 13 US academic centers. This study was approved by the institutional review board of all involved sites. We identified adult patients diagnosed with r/r aggressive B-NHL treated with CD19 CAR-T therapy between 1 January 2015 through 25 December 2021. Variables examined included baseline demographics, insurance type, and clinical data. Demographic data included age at time of treatment, sex, and race. Race was stratified between Caucasian, AA, Asian, or other. We excluded patients categorized as “other” from our analysis because there were only 2 patients. Ethnicity was identified as either Hispanic or non-Hispanic. Both race and ethnicity were extracted from electronic medical records.
To evaluate clinical factors and health care use patterns that could affect outcomes, we assessed median lines of therapy before CAR-T therapy, exposure to autologous SCT before CAR-T therapy, median time from diagnosis to initiation of CAR-T therapy, median time from most recent relapse or progression to initiation of CAR-T therapy, time from apheresis to CAR-T infusion; use of bridging therapy, type of CAR-T construct used, administration of CAR-Ts on clinical trial, and administration of therapy after failure of CAR-T treatment. Insurance type was categorized as either Medicare, Medicaid, private insurance, or self-pay. Outcomes that we analyzed were rates of toxicity including cytokine release syndrome and immune-effector cell associated neurotoxicity syndrome, overall response rate (ORR), complete response (CR) rate, PFS, and OS.
Baseline demographics, insurance type, and clinical data were compared across race and analyzed using χ2 tests. PFS and OS were estimated using Kaplan-Meier analysis and their differences assessed by log-rank test. A Cox multivariable regression was used to analyze the impact of race, ethnicity, and other clinical and demographic variables on PFS and OS. Variables used in the multivariable analysis included race, age, insurance type, measures of disease burden (lactate dehydrogenase [LDH] level and International Prognostic Index score at time of apheresis), and parameters of health care use including prior autologous SCT, CAR-T therapy administration on clinical trial, and use of bridging therapy to address our study objective.
The institutional review board from Northwestern University approved the study.
Results
Patient characteristics and measures of health care use before CAR-T therapy
In total, 466 adult patients were included in our analysis. Table 1 outlines demographic and clinical data, and parameters of health care use for our patients. Only patients with de novo diffuse large B-cell lymphoma or transformed follicular lymphoma were included for analyses. Overall, 406 (87%) patients were Caucasian, 34 (7%) AA, and 26 (6%) Asian; 9 (2%) patients were Hispanic, all of whom identified as Caucasian. Caucasians were older than AAs and Asians (median age, 59 vs 55 vs 55 years, respectively; P = .004). There was no significant difference in median number of lines of therapy before CAR-T therapy by race (P = .44). However, Caucasian patients were more likely to have had prior autologous SCT (P = .04).
For Caucasian vs AA vs Asian patients: median time from last relapse/progression before CAR-T therapy to CAR-T therapy was 2 vs 1.8 vs 1.2 months, respectively (P = .9); rates of bridging therapy were 44% vs 61% vs 46%, respectively (P = .17); and rates of use of clinical trials for CAR-T access were 29% vs 15% vs 19%, respectively (P = .12). Median time from apheresis to CAR-T therapy was 1.1 vs 1.1 vs 0.9 months, respectively (P = .2). Asian patients had a significantly higher rate of LDH elevation at time of CAR-T collection (83%) than Caucasian (54%) and AA (41%) patients (P = .005). There was no difference in rates of grade ≥3 cytokine release syndrome or immune-effector cell associated neurotoxicity syndrome across race (P = .8 and P = .4, respectively).
Table 2 outlines clinical characteristics of our patients stratified by insurance type. Of note, we did not have any patients with non-Medicare/Medicaid forms of governmental insurance in our data set. Self-pay patients predominantly consisted of international patients traveling to centers in the United States for CAR-T therapy. There was no significant difference between types of insurance coverage (P = .09) among races. As to be expected, Medicare patients were found to be significantly older than patients with other insurance types (64.0 years for Medicare vs 52.5 years for Medicaid vs 56.0 years for private vs 56.0 years for self-pay; P < .001). Medicare patients also had a significantly longer time from initial diagnosis to CAR-T therapy initiation (26.2 months) vs Medicaid (14.4 months) vs private (14.0 months) vs self-pay (14.5 months; P = .001). There was no difference in rates of bridging therapy between insurance types (P = .166), time from last progression to CAR-T therapy (P = .067), and LDH elevation at time of apheresis (P = .979).
Response rates and next-line treatment
Caucasian and AA patients had a higher day-180 ORR than Asian patients (51% vs 46% vs 19%, respectively; P = .04). However, there was no difference in day-180 CR rates between races (43% vs 42% vs 19%, respectively; P = .15).
After progression, practice patterns for next-line therapy were compared. The most commonly used next-line therapies included chemotherapy, radiation, immunomodulatory agents, checkpoint inhibitors, and polatuzumab-bendamustine-rituximab. For these salvage regimens, there was no difference in rates of use between races (P = .812). However, Caucasian patients trended toward having further lines of therapy after progression after CAR-T therapy when compared with AA and Asian patients (46% vs 25% vs 28%, respectively; P = .05). Salvage therapies after CAR-T administration did not differ significantly between insurance groups (P = .683).
Survival outcomes
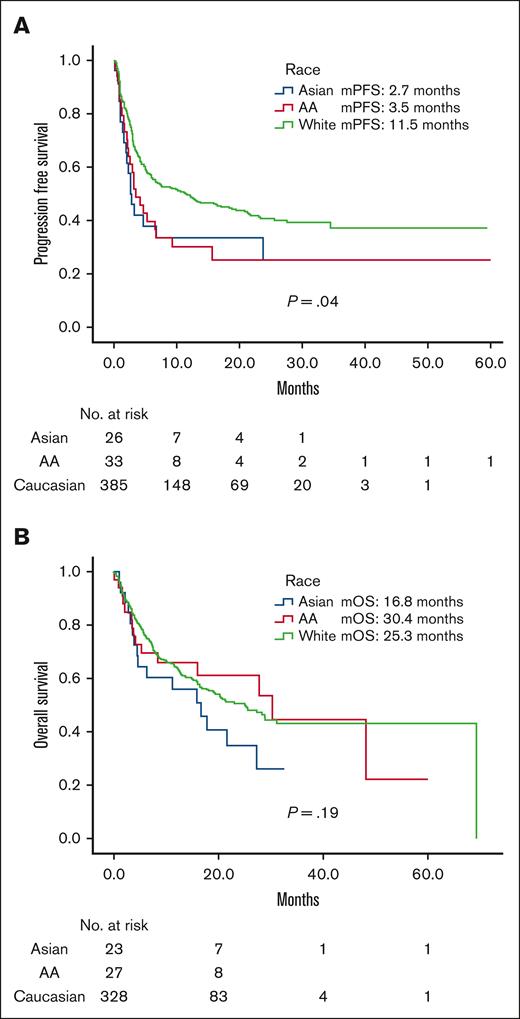
Median follow-up time was 12.7 months for our cohort. Median PFS (mPFS) was longer for Caucasian (11.5 months) than for AA (3.5 months; hazard ratio [HR], 1.56 [1.03-2.4]; P = .04) or Asian (2.7 months; HR, 1.7 [1.02-2.67]; P = .04; Figure 1A) patients. Differences in median OS (mOS) were not statistically significant across race (Figure 1B). Median OS (mOS) was 25.4 months in Caucasian vs 30.4 months in AA (HR, 1.0 [0.59-1.69]; P = .99) vs 16.8 months in Asian (HR, 1.42 [0.84-2.42]; P = .19) patients.
Survival stratified by race. (A) Progression free survival. (B) Overall survival. Median follow-up time 12.7 months.
Survival stratified by race. (A) Progression free survival. (B) Overall survival. Median follow-up time 12.7 months.
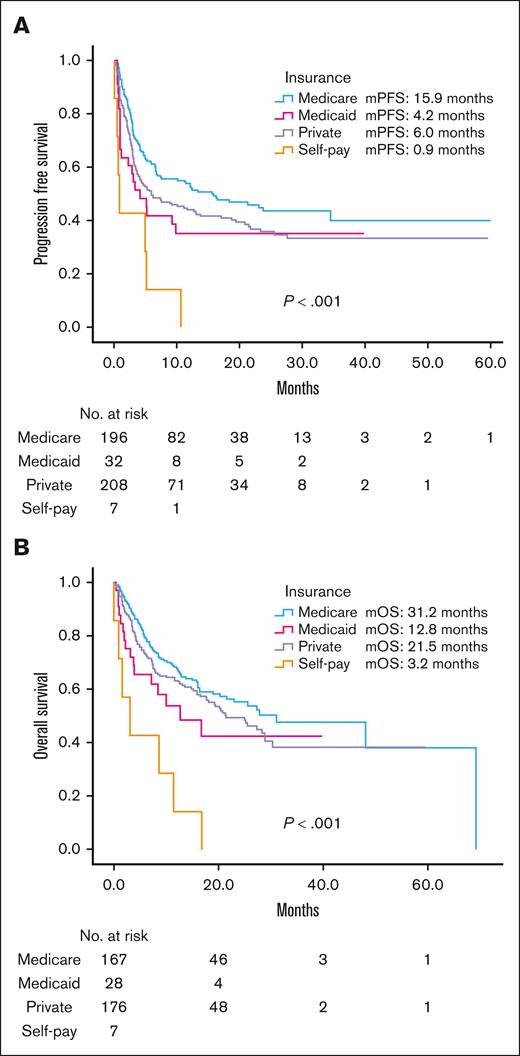
There were significant differences in mPFS and mOS between payer group (Figure 2). For Medicare (n = 206) vs Medicaid (n = 33) vs private insurance (n = 219) vs self-pay (n = 7): mPFS was 15.9 vs 4.2 vs 6.0 vs 0.9 months, respectively (P < .001); and mOS was 31.2 vs 12.8 vs 21.5 vs 3.2 months, respectively (P < .001).
Survival stratified by insurance type. (A) Progression free survival. (B) Overall survival. Median follow-up time 12.7 months.
Survival stratified by insurance type. (A) Progression free survival. (B) Overall survival. Median follow-up time 12.7 months.
Table 3 outlines our multivariate analysis of PFS and OS in our patients. Race was found to affect PFS (P = .03), with AA patients having significantly worse PFS than Caucasian patients (HR, 1.72 [1.05-2.82]; P = .03). Asian patients did not have a significantly worse PFS as compared with Caucasian patients (HR, 1.59 [0.94-2.7]; P = .08). Race overall did not affect OS (P = .10). Insurance was found to affect both PFS and OS, with Medicare coverage having a positive impact on PFS and OS compared with patients with alternative insurances payors (P < .001 and P < .001, respectively). Medicare when compared individually to each insurance type showed significantly improved OS compared with Medicaid (HR, 3.23 [1.55-6.72]; P = .002) and self-pay (HR, 3.51 [1.44-8.54]; P = .006) but not private insurance (HR, 0.94 [0.64-1.38]; P = .76). Use of bridging therapy also negatively affected both PFS (HR, 1.59 [1.19-2.12]; P = .002) and OS (HR, 1.51 [1.06-2.15]; P = .02). LDH elevation at apheresis negatively affected PFS (P = .001) but was not significantly associated with worse OS (HR, 0.91 [0.65-1.27]; P = .57). CAR-Ts received on clinical trial was associated with inferior OS (HR, 1.47 [1.01-2.14]; P = .04). Receiving additional therapy after CAR-Ts was associated with improved OS (HR, 0.64 [0.46-0.9]; P = .01).
Discussion
Our data represent, to our knowledge, the first to analyze the impact of both race and other social determinants of health on patients with r/r aggressive B-NHL treated with CAR-Ts. Despite being a multicenter study with several sites having an urban catchment, the majority of our patient population was Caucasian (87%). AA and Hispanic patients were underrepresented in our patient data set. AA patients only made up 7% of our treated patients, whereas Hispanic patients made up 2%. To provide context, historically for B-NHL, non-Hispanic Caucasian patients have the highest incidence of disease (24.7 and 15.8 cases per 100 000 persons for male and females, respectively) compared with AA patients (17.4 and 12.4 cases per 100 000 persons for male and females, respectively) and Hispanic patients (20.2 and 15.3 cases per 100 000 persons for male and females, respectively).13 However, these incidences do not account for the substantially disproportionate underrepresentation of minorities in our data set.
Unfortunately, our data set is not unique in this regard. Locke et al analyzed patients with large B-cell lymphoma receiving axicabtagene ciloleucel and compared outcomes between racial groups in the real-world setting; the aforementioned study had similar proportions of AA (5%) and Asian (6%) patients as our study, although they did capture a higher percentage of Hispanic patients (11%).14 Receipt of care at underresourced centers with limited access to newest therapy and clinical trials may explain decreased representation of racial minorities in studies like ours.4 Furthermore, patients included in this study were treated at major US academic institutions, and although most centers were in urban settings, some data suggest that racial minorities can be less represented at major academic centers.15
In our cohort as a whole, we previously reported survival outcomes consistent with results seen on prospective studies.16 However, our multivariable analysis suggested discrepant findings in PFS and OS by race. Asian patients did have significantly lower ORRs when compared with Caucasian and AA patients, which did not translate to significant differences in neither PFS nor OS. AA patients had significantly worse PFS than Caucasian patients but significantly improved OS. These discrepancies may be attributed to sample size. These findings may also be attributed to the fact that Caucasian patients were older than their racial counterparts. Interestingly, there was no difference between racial groups when it came to resource use. Specifically, time from initial diagnosis to CAR-T therapy, time from last relapse to CAR-T therapy, number of prior lines of therapy, bridging therapy, rates or toxicities, and types of salvage regimens used after progression, were similar between racial groups and suggest against any of these modifiable factors as being responsible for differences in outcomes. That being said, our results do not capture patients facing insurance denial and/or poor access that would likely augment trends for racial and socioeconomic disparities identified in our CAR-T population. Although difficult to prove in this analysis, a biologic impact from race could have also affected these outcomes, as has been shown in treatments for other conditions.17
Notably, our analyses did show a survival benefit overall for patients who received additional therapy after CAR-T therapy (P = .01). However, we also identified a trend toward receiving further therapy after disease progression after CAR-Ts in Caucasian patients (P = .05), which did not translate to a benefit in OS for Caucasian patients as compared with patients of other races. To our knowledge, only 1 other analysis has investigated CAR-T outcomes in B-NHL between races. This analysis did show that Caucasian patients had improved ORR and CR when compared directly with AA patients, but this was not associated with differences in survival between the racial groups.14
Our study also investigated the impact of insurance on outcomes with CAR-Ts and showed that patients on Medicare had the best outcomes. Medicare patients in our cohort may have had the advantage of having less aggressive disease, as suggested by their significantly longer time from diagnosis to CAR-T therapy initiation; this may have led to the more favorable outcomes seen in the cohort in our analysis. Our observations are complementary to what has been reported in the literature, traditionally Medicaid patients have overall worse outcomes in oncology compared with patients with other insurances, which has mainly been attributed to patients presenting with more advanced or aggressive disease, which makes them ineligible for curative therapies, although Medicaid patients are often younger.18,19
The majority of our self-pay patients were international patients traveling to the United States seeking out CAR-T treatment. As a result, these patients typically had a trend for longer times from progression to CAR-T therapy (P = .067). Longer times from progression to CAR-T infusion, termed “brain-to-vein” time are felt to affect outcomes negatively among CAR-T providers.20 As a result, it is likely that self-pay patients had higher tumor burden before CAR-T therapy, a factor known to impact progression after CAR-Ts.21,22 Notably, Medicare patients also had statistically significant longer times from diagnosis to CAR-T infusion of 26 months. Patients with other payor types typically relapsed within ∼12 months of their diagnosis, in keeping with primary refractory disease. This suggests that Medicare patients may have had less aggressive disease biology to start, another factor that may have potentially contributed to better survival in this payor group. Inferior survival in primary refractory disease has been observed by other investigators and supports this postulation.23
Of note, distance to treating center was not collected in our data set. This is an important variable in understanding impact on access to care, insurance type, and outcomes according to geographical distribution of race. Future studies addressing impact of social determinants of health on clinical outcomes should address this question. Taking things further, CAR-T centers should be incentivized to focus on data collection and performance measurement unique to each organization to better identify the multifactorial core issues driving disparities in quality of care for racial and ethnic minorities across diverse geography.
In conclusion, our multicenter retrospective analysis showed that race and social determinants of health can influence treatment outcomes with CAR-T therapy. Our data show that there are real disparities in who receives CAR-Ts for aggressive B-NHL by race. Additionally, we found that Medicare patients had improved outcomes compared with other payor types, although this was likely confounded by Medicare patients having less aggressive disease in this data set. Undoubtedly, in data sets such as ours, the inability to capture patients with poor access as a result of distance to treating centers or other causes, or insurance denial, poses a barrier to analyses and would likely augment findings of disparities. However, such data sets serve to raise awareness of racial and ethnic disparities in health care among clinical providers and the general public, which is a critical step toward reducing disparities in health care. Future prospective studies are needed to better understand the causation for these effects. The interaction between race and insurance status and their relative contributions to access and outcomes with CAR-T therapy should be further explored. It will also be imperative for future prospective trials and studies to include better representation from racial minorities to confidently guide treatment strategies and decrease treatment disparities.
Authorship
Contribution: R.M. and R.K. conceptualized and designed the study; all authors collected, assembled, and interpreted the data; R.M., I.R., and R.K. analyzed data; R.M. and R.K. prepared the first draft of the manuscript; and all authors provided critical and insightful comments and approved the final version of the manuscript.
Conflict-of-interest disclosure: N.E. received research funding from BeiGene; serves on the speakers bureau for BeiGene, Incyte, and Novartis; received honoraria, consulted for, and served on advisory boards of, Merck, ADC Therapeutics, Ipsen, Lilly, and Novartis. N.N.S. reports consultancy or advisory committee participation with Miltenyi Biotec, Lilly Oncology, Bristol Myers Squibb/Juno, Galapagos, Gilead/Kite, AbbVie, Incyte, and Seattle Genetics; received research funding from both Miltenyi Biotec and Lilly Oncology; and serves on the scientific advisory board for Tundra Therapeutics. D.M.S. has received consulting fees from AbbVie, AstraZeneca, BeiGene, Bristol Myers Squibb, Celgene, Eli Lilly, Genentech, and Janssen; and has received institutional research funding from AstraZeneca and Novartis. T.O. received research funding from Loxo Oncology; received consulting fees and research funding from ONO Pharmaceuticals; and received consulting fees from ADC therapeutics. G.S. serves on the advisory boards of Kite, BeiGene, and AstraZeneca; and reports speakers bureau participation for Kite and BeiGene. A.D. has received consulting fees from AbbVie, AstraZeneca, BeiGene, Bristol Myers Squibb, Genentech, GenMab, Incyte, Janssen, Lilly Oncology, MEI Pharma, Merck, Nurix, and Prelude; and has ongoing research funding from AbbVie, AstraZeneca, Bayer Oncology, BeiGene, Bristol Myers Squibb, Cyclacel, GenMab, Lilly Oncology, MEI Pharma, MorphoSys, and Nurix. P.T. declares consultancy for TG Therapeutics, ADC Therapeutics, Genentech, GenMab, Seagen, and Lilly Oncology. S.K.B. received honoraria from Acrotech, Affimed, Daiichi Sankyo, Kyowa Kirin, Janssen, and Seagen. J.B.C. declares consultant/adviser role with AstraZeneca, AbbVie, BeiGene, Janssen, Loxo/Lilly, Kite/Gilead, and ADC Therapeutics; and received research funding from the Leukemia and Lymphoma Society, Genentech, AstraZeneca, Novartis, Loxo/Lilly, and Bristol Myers Squibb/Celgene. J.R. received research funding from Merck, Corvus Pharmaceuticals, and Kymera Therapeutics; and reports a consulting role with Acrotech Biopharma and Kyowa Kirin. J.N.W. reports research funding and honoraria from Merck; reports research funding to spouse from Cellectis, Daiichi Sankyo, Rafael, Forty Seven/Gilead, and Astellas; received research funding and consultancy fees to spouse from Novartis; and reports consultancy fees to spouse from Astellas, Ariad/Takeda, CVS/Caremark, Curis, Jazz Pharmaceuticals, King Biotherapeutics, and Rigel Pharmaceuticals. S.M. received research funding from AbbVie, AstraZeneca, BeiGene, Janssen, Juno, Loxo, and TG Therapeutics; reports speakers bureau participation with AstraZeneca, BeiGene, and, Lilly; and served on the advisory boards of AbbVie, AstraZeneca, Bristol Myers Squibb, Genentech, and Janssen. R.K. serves on the advisory board of Bristol Myers Squibb, Gilead Sciences/Kite Pharma, Janssen, MorphoSys/Incyte, Genentech/Roche; received grants/research support from Bristol Myers Squibb, Takeda, BeiGene, Gilead Sciences/Kite, and Calithera. Speakers Bureau: BeiGene, AstraZeneca, MorphoSys/Incyte. The remaining authors declare no competing financial interests.
Correspondence: Reem Karmali, Division of Hematology and Oncology, Northwestern University Feinberg School of Medicine, 676 N. St Clair St, Suite 850, Chicago, IL 60611; email: reem.karmali@northwestern.edu.
References
Author notes
Data are available on request from the corresponding author, Reem Karmali (reem.karmali@northwestern.edu).