Key Points
A qualitative study of patients in remission from iTTP documented nearly universal occurrence of cognitive problems, fatigue, and anxiety.
These sequelae of iTTP caused problems with careers, social activities, family responsibilities, and marital relationships.
Abstract
The impact of residual symptoms following recovery from immune-mediated thrombotic thrombocytopenic purpura (iTTP) on activities of daily living during remission is not routinely discussed or evaluated by hematologists. This study used qualitative methodology to understand 3 issues from the patient’s perspective: the most important symptoms during remission, the impact of these symptoms on their daily activities, and the effectiveness of communication with hematologists. Oklahoma and Ohio patients participated in either focus groups or individual interviews. Eligibility included age ≥18 years, ADAMTS13 deficiency (<10% activity) at diagnosis or relapse, and in clinical remission (≥1 year from episode). A nonprobabilistic purposive sampling approach was used. The most important symptoms were defined as symptoms mentioned across all 7 focus groups. The interviews supplemented focus group data. The analysis focused on describing the impact of symptoms and barriers to communicating with hematologists. A total of 44 patients participated (focus groups, N = 25; interviews, N = 19). The most important symptoms affecting the patients’ daily activities were cognitive issues, anxiety, depression, and fatigue. These symptoms affected patients’ ability to return to their previous level of functioning and created difficulties in relationships. A key communication barrier with their hematologists was forgetting to mention these symptoms. Although hematologists pronounce patients as recovered, iTTP remains a life-changing event. Patients often did not return to their previous functioning; relationships and careers were affected. However, patients may forget to discuss these concerns with their hematologist. To improve remission care, hematologists should incorporate patient-reported outcome measures evaluating these symptoms in remission visits.
Introduction
Immune-mediated thrombotic thrombocytopenic purpura (iTTP) is an uncommon disorder.1 With prompt recognition and treatment, >90% of patients will survive beyond their acute episode.2-4 Furthermore, the last decade has witnessed enormous progress toward anticipating and preventing relapse.5
Notwithstanding these significant advances in management, the sequelae of iTTP can cause critical problems. The Oklahoma Registry was the first to demonstrate that following recovery from an acute episode, patients have long-term risks for cognitive impairment,6,7 major depression, hypertension, systemic lupus erythematosus,8 and a shortened life expectancy.8 Multiple subsequent studies have confirmed the frequency and severity of cognitive impairment and depression.9-12 Depression and cognitive impairment in patients with iTTP often remain unrecognized and untreated,11 and patients often believe their depression is not severe enough to warrant any treatment.13
These previous studies have not assessed the impact of residual symptoms on activities of daily living. Indicators of social health include the ability to participate in, and satisfaction with, one’s social role.14 Social roles include marital relationships, family responsibilities, and responsibilities as a worker, student, or member of a community.15 This is significant as a lack of a social connection is associated with an increased risk of premature mortality,16 and satisfaction with one’s social activities is an important aspect of overall health.17 Here, to describe the patient’s perspective of high-impact symptoms during remission and to identify previously unrecognized residual symptoms, we used qualitative methodology. In addition, we described the impact of these symptoms in the following ways: (1) identified barriers to communicating residual symptoms to their hematologists, (2) described the impact of residual symptoms on returning to work, and (3) described the impact of symptoms on relationships. The primary goal of this study was to determine, from the patient’s perspective, the high-impact symptoms that are necessary to evaluate during remission. A secondary goal was to identify barriers to patient-hematologist communication related to residual symptoms during remission.
Methods
Study design
Focus groups and individual interviews were used to identify important residual symptoms during remission. Our initial assumption was that these symptoms were not sensitive, making focus groups appropriate. However, individual interviews were later added as an option for patients who were either unable to attend a focus group or were uncomfortable with group discussion.18 This also provided an opportunity to identify sensitive topics and/or symptoms that patients would not feel comfortable discussing with others.19,20 Patients received a reminder telephone call 1 to 2 days before the scheduled focus group or interview.
Patients
The Oklahoma TTP Registry began in 1995 when serum samples were collected from all patients at the time of their initial plasma exchange. It is a prospective, population-based inception cohort of all consecutive patients within a defined geographic region.21 The Ohio State University (OSU) TTP Research program began in 2003. The OSU TTP Research program includes patients with iTTP who are referred to and treated at OSU.
Patients were recruited from the Oklahoma Registry and the OSU cohort. Eligibility included: (1) age ≥ 18 years, (2) documented ADAMTS13 deficiency (<10% activity) at diagnosis or during a relapse, (3) being in clinical remission from iTTP (at least 1 year from the last episode), and (4) able to read and understand English.
A nonprobabilistic purposive sampling approach was used to select “information-rich” patients from whom the most could be learned.22 The goal was to understand the post-iTTP lived experience of patients. Therefore, to understand the full range of health challenges during clinical remission, we intentionally included at least 1 patient who had previously experienced a relapse (at least 1 year ago) in each focus group.
From the Oklahoma Registry, all patients who met eligibility criteria were given identification numbers. To avoid selection bias, we used a simple random sample program (SAS version 9.4; SAS Institute, Cary, NC) to select patient identification numbers to approach for participation. Patients were contacted by the coordinator and described the purpose of the study. After verbal consent, consent forms and additional information were mailed to the patient. Following the amendment allowing the option of individual interviews, patients who declined or were unavailable for a focus group were asked to participate in an individual interview. Once the prespecified number of focus groups were completed, patients were only recruited for individual interviews.
OSU patients were given an institutional review board (IRB)–approved flyer, and interested patients were instructed to contact the Oklahoma principal investigator. The process following verbal consent was the same as described above.
Focus groups and individual interviews
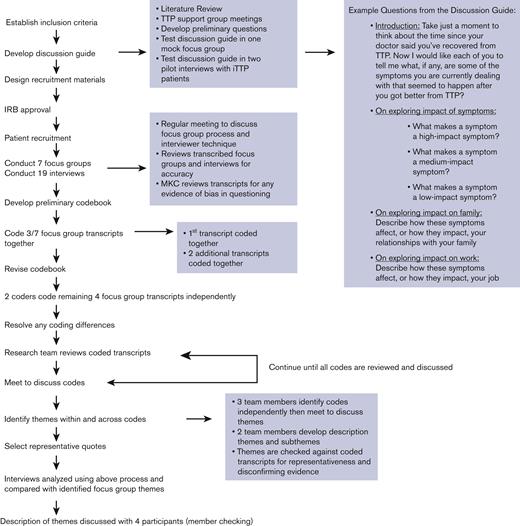
A semistructured discussion guide was used to facilitate the discussion. The discussion guide was developed based on a comprehensive literature review along with feedback from patients in the Oklahoma Registry, collected during support group meetings. The discussion guide was pilot tested for question flow and understandability in a mock focus group of people without iTTP and in 2 pilot interviews with patients with iTTP from the Oklahoma Registry and revised accordingly (Figure 1). Data from the pilot tests were not used in the analysis.
To improve the rigor of the study and to provide consistency, 1 moderator conducted all focus groups and interviews. Although most of the Oklahoma Registry patients had met the moderator previously, none of the Ohio patients knew the moderator. The research team reviewed the transcripts to assess for any patterns in differences in data collection between the 2 sites. These reviews did not reveal any such differences between the sites, so the data from both sites were pooled for analysis.
Patients were informed of the goal of the study before the start of the focus group or interview. Focus groups were conducted in-person and consisted of 2 to 5 patients each (4 focus groups at the University of Oklahoma Health Sciences Center in Oklahoma City, Oklahoma; 3 focus groups at the James Cancer Center in Columbus, Ohio). Individual interviews were conducted in-person, over the telephone, and over computer applications. To assist with the activities and to take notes to enhance the accuracy of the transcripts, 2 assistant moderators were present for the focus groups, and 1 assistant was present for the interviews. Focus groups and interviews were conducted until no new content was heard related to our key questions of interest, which is defined as data saturation.23 As an additional method of data validation, 4 patients (2 interviewed patients from Ohio; 2 focus group patients from Oklahoma) were sent a summary of the results and asked to comment if the summary was consistent with their experience (ie, member checking).
During the focus groups and interviews, patients described their symptoms following recovery from iTTP. Patients were probed about symptoms previously identified from the literature and Oklahoma Registry experience if they were not mentioned spontaneously.24 Each mentioned symptom was then transferred to multicolored index cards by an assistant moderator and given to each patient. Patients who participated in virtual interviews were mailed a packet of multicolored sticky notes before their interview, and during the interview were asked to record their symptoms onto the sticky notes. Patients were then asked to individually sort symptoms/conditions into categories (“high,” “medium,” “low,” or “not sure”) based on importance, severity, and the degree of interference with their normal daily activities. Following the sorting activity, patients discussed what made a symptom high, medium, or low impact. In addition, patients were given a white index card and instructed to individually list their top 3 symptoms, which had the most impact or were the most bothersome on their daily life (regardless of whether it had been previously mentioned aloud). Patients were informed that the white index card with the individual top 3 symptoms would not be discussed aloud to allow privacy for patients to report sensitive concerns.
Analysis
We used the Consolidated Criteria for Reporting Qualitative Research checklist to promote a comprehensive report of the methods and findings.25 Each focus group and interview was transcribed verbatim. Content analysis was conducted by 3 researchers trained in qualitative research methodology using the QSR NVivo 12.0 software. Initially, focus group data were analyzed. Three researchers read each transcript, developed a preliminary codebook, and coded 1 focus group transcript together. Then 2 team members independently coded 2 focus group transcripts and met to resolve differences. Then 2 coders independently coded the remaining transcripts (interrater reliability percent agreement 97.9%). The research team met to review and resolve any coding differences (Figure 1).
The same codebook was used to code the interview data. Interview data was used to supplement focus group data and identify any differences between patients who participated in focus groups compared with individual interviews (Figure 1).
The most prevalent symptoms were those that were mentioned across all the focus groups. These symptoms were then cross-compared with the top 3 symptoms listed on the individual white index cards.
Qualitative analysis focused on what made a symptom have a high impact on a patient’s daily life and whether these symptoms affected their ability to return to work or their relationships. Analysis also focused on barriers to communicating these symptoms to their hematologist.
Compensation
All patients received a $50.00 VISA gift card. Patients were reimbursed for mileage at the standard Internal Revenue Service rate if they traveled >40 miles roundtrip to participate.
Ethics approval
Informed consent was given in accordance with the Declaration of Helsinki. Patients consented to the focus groups or interviews being digitally recorded. The study was approved by the ethics committee at the University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma (OSU IRB ceded to the University of Oklahoma Health Sciences Center; IRB #9133).
Results
A total of 44 patients were included in the study (25 patients participated in focus groups; 19 participated in individual interviews). Focus groups were 54 to 94 minutes in duration (May-September 2018). The individual interviews were 21 to 73 minutes in duration (September 2018-June 2019).
The gender and age profile of the participants is similar to patients with iTTP in general. Most of the patients were female (76% female focus groups; 84% female individual interviews), and the median age was 46 years for the focus groups and 51 years for the interviews. The highest level of education obtained was less than a college degree for most of the patients (76% focus groups; 63% individual interviews). Most of them (52% focus groups; 68% individual interviews) had experienced an iTTP relapse and were more than 5 years from their most recent episode (80% focus groups; 53% individual interviews) (Table 1). A total of 14 (5 focus group; 9 individual interview) patients self-reported they had experienced a stroke during their lifetime (this included before diagnosis and/or following recovery from iTTP).
In all 7 focus groups, patients reported dealing with residual symptoms following iTTP episode(s). The most important symptoms (mentioned in all focus groups) included cognitive concerns, fatigue, and anxiety. Depression was mentioned in 6 out of 7 focus groups. In addition, the top 3 symptoms focus group participants anonymously recorded on their individual index cards were: cognitive (19/25; 76%), fatigue (17/25; 68%), and depression or anxiety (9/25; 36%).
Of the patients interviewed, 18 of 19 (95%) were dealing with residual symptoms following iTTP episode(s). Of the interviewed patients, 9 self-reported having experienced a stroke. Although no symptom was mentioned in every interview, 17 out of 19 (89%) patients described dealing with residual cognitive concerns, 14 (74%) described fatigue, and 13 (68%) described anxiety. Unlike focus group participants, the top 3 symptoms recorded on the white index cards for interviewed patients were not anonymous. Overall, the top 3 symptoms were: fatigue (10/19; 53%), cognitive concerns (9/19; 47%), and depression or anxiety (10/19; 53%). This was consistent in both patients who were less than or more than 5 years from their last episode. The top-reported symptoms for the 9 patients who had experienced a stroke were consistent with the overall results: 6 out of 9 (67%) listed depression or anxiety, 4 (44%) listed cognitive concerns, and 4 (44%) fatigue.
Patient descriptions of residual symptoms
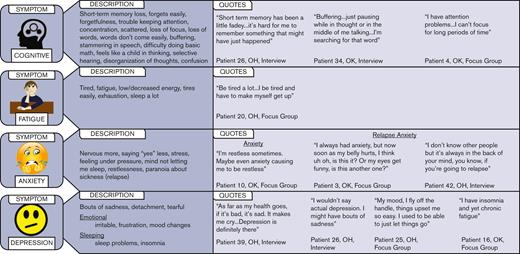
The cognitive difficulties during remission were challenging for patients to describe, and the terms included: short-term memory loss, forgetting easily, scattered, buffering, words don’t come easily, disorganization of thoughts, confusion, and feeling like a child in thinking. Most of the patients described fatigue as being tired or being exhausted. Descriptors for anxiety included restlessness, feeling under pressure, and the feeling of being more nervous now than before their iTTP episode. In addition to general anxiety, there was anxiety specifically related to fears of an impending iTTP relapse. Some patients described a constant fear of a relapse, daily monitoring symptoms such as stomach pain, the color of their urine, and how their bodies felt so they could detect if a relapse was about to happen. Although many patients used the word depression, some patients refused to describe themselves as being depressed and instead used terms such as “bouts of sadness.” Figure 2 shows the associated patient descriptions and illustrative quotes for the most important symptoms (ie, cognitive, fatigue, anxiety, and depression).
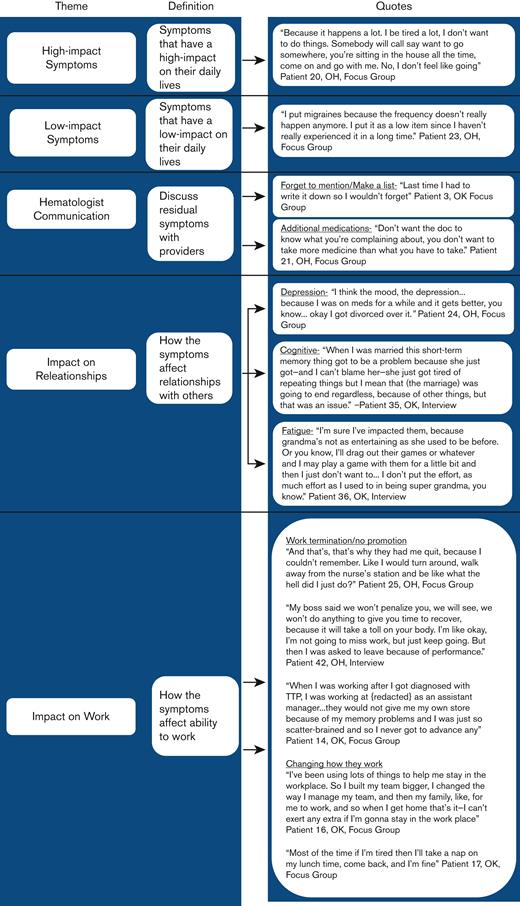
In addition to the most important symptoms, there were 9 other categories of residual symptoms mentioned by patients. In 6 out of the 7 focus groups and in 5 out of the 19 interviews, patients described an inability to return to pre-iTTP activity/social roles in their marital relationships, family responsibilities, at work, and social activities. To a lesser extent, patients also described vision problems and migraines/headaches, sensory problems (body aches, numbness in body), physical changes, hair changes, changes in weight, lung problems, changes in sexual intimacy, fears related to pregnancy, and claustrophobia (Table 2).
Patients also described what differentiated high-impact vs low-impact symptoms, discussed any barriers they encountered in communicating symptoms during remission appointments, and discussed the impact of symptoms on their ability to return to life following recovery from an iTTP diagnosis.
Patient’s perspective: residual high-impact symptoms
Patients stated both frequency and severity were important in determining the impact of symptoms on their activities of daily living. Symptoms classified as having a high impact were not easily relieved by medication or other interventions. For example, fatigue was listed as a high-impact symptom, and patients described feeling tired almost daily despite interventions (ie, increased sleep, exercise, or medication for insomnia). Alternatively, the symptom of a headache was often described as a medium or low-impact symptom because patients stated that their headaches did not occur daily, or when they did occur, they were relieved by interventions such as medication, sitting in the dark, or resting (Figure 3).
Patient-hematologist communication during remission
In 5 of the 7 focus groups, patients reported their hematologist asking about cognitive concerns, fatigue, and depression during remission visits. In addition, in 5 of the 7 focus groups, patients described that their hematologist recognized they were suffering from depression and either placed them on medication or suggested that they speak with a mental health specialist. In addition, patients in 3 focus groups described that their hematologist acknowledged residual issues of fatigue and cognition by either telling them how these issues would not get any better or letting them know that other patients were also dealing with these concerns.
Yet, barriers to communicating with their hematologist about residual issues were also described in the focus groups and throughout the individual interviews. In 6 of the 7 focus groups, patients stated the main barrier to discussing residual issues was forgetting to mention them. Patients described needing to make lists before their visit as a reminder to communicate memory, concentration, and fatigue concerns (Figure 3). In 3 of the 7 focus groups, patients described a barrier where the patient was worried if they mentioned a concern to their hematologist that the hematologist would prescribe additional medication (Figure 3). In addition, in 3 of the 7 focus groups, patients described how they felt it was unnecessary to speak with their hematologist about residual issues because they had essentially reframed their expectations of what their life should look like following recovery and therefore had adjusted to the residual issues being present. In 1 focus group, patients described they felt like it was complaining to mention fatigue or memory issues when their hematology laboratory results were normal and their hematologist told them they were healthy. In addition, in 1 focus group, patients said they restricted discussion with their hematologist to concerns regarding iTTP and talked to their primary care doctor about everything else.
Patient perspective: residual symptom impact on relationships
In 4 of the 7 focus groups and throughout the individual interviews, patients reported their residual symptoms affected relationships with others. In 3 of the 7 focus groups, patients stated they were not the same person post-iTTP as they were pre-iTTP. Specifically, following acute episodes, patients describe themselves as being more short-tempered, having less patience, and being more argumentative than pre-iTTP. Challenges related to memory, concentration, and attention also led to tension in relationships with others, especially when instructions, details, or previous conversations needed repeating. In 1 focus group and 1 interview, patients stated depression, personality changes, and/or cognitive problems following iTTP ultimately contributed to their divorce (Figure 3). In addition, fatigue kept patients from enjoying activities and hobbies they enjoyed before their iTTP diagnosis, such as playing with their grandchildren (Figure 3). Fatigue also affected self-esteem by creating a sense of lack of motivation, resulting in patients describing themselves as “lazy” or “inadequate.”
Patient perspective: returning to work following recovery
Following clinical recovery from iTTP, hematologists advised patients to return to their previous activities. However, in 5 of the 7 focus groups and throughout the individual interviews, patients stated that residual symptoms significantly affected their ability to return to their previous employment and perform their job at the same level. For some patients, the inability to remember important details while they were at work, caused them to either quit working completely, choose to take an early retirement, or change occupations. One patient was fired following an iTTP relapse, and their employer stated it was owing to poor performance (Figure 3).
Patients who returned to their previous employment described using various coping mechanisms to continue employment. In addition, patients in 3 of the 7 focus groups stated that before iTTP they were being offered promotions, but after they returned to work following their episode they were either informed they were no longer being considered for the promotion or they turned down the opportunity because they no longer felt comfortable taking on additional responsibilities (Figure 3).
Discussion
Our data from the patient’s perspective describe what may be the most important residual symptoms following recovery from iTTP. Their cognitive issues, fatigue, depression, and anxiety are consistent with our a priori hypothesis.7-12 However, the impact of residual symptoms on remission and social health are aspects of iTTP not previously described. Knowledge from our data provides hematologists and primary care physicians with information that can help them recognize and work to create patient discussions of these significant aspects of their health. Using standardized patient-reported outcome instruments to assess objectively what patients’ cognitive ability, fatigue, and symptoms of depression are can help providers overcome barriers patients identified in communicating these concerns with their providers. Using this process provides a universal, systematic method that anticipates patients’ social, family, and work difficulties.
Being aware that iTTP is a life-changing event with a significant ongoing impact on the health and well-being of individuals is critically important. It is important to recognize that patients often do not return to previous functioning but instead have learned to function with life-changing residual impacts of the disease. Social connection is multifactorial and includes both perceived and received support along with structural components. Here, patients with iTTP described that they were less likely to want to socialize following recovery and had an increased tendency to isolate themselves from others. Patients also described themselves as more short-tempered, impatient, and argumentative following recovery. In addition, fatigue limited patients from spending time with their loved ones and affected their self-esteem.
This study was the first to qualitatively describe, from the patient’s perspective, the impact of residual symptoms on daily lives. Our results are consistent with the limited studies in this area. In a recent study of patients in the United Kingdom, 43% of patients with iTTP reported a decrease in work productivity following recovery.26 In this same study, most of the patients reported that fatigue affected their family life, social activities, and work, and 84% of patients were worried about having a relapse.26 A previous study reported that a third of iTTP survivors met the criteria for posttraumatic stress disorder.12
The limitations of our study include recruitment of patients from 2 sites (Oklahoma and Ohio) that focus on researching long-term outcomes of iTTP; therefore, some of the patients may have been discussing these symptoms with their hematologists before our study. In contrast, most of our patients’ highest level of education was less than a college degree, which could have affected their comfort with communicating these symptoms with their providers. An additional limitation is that the residual symptoms were collected via patient self-report and not verified from medical records or medication history. However, post hoc, we were able to verify that in 8 of 9 patients from the Oklahoma Registry who self-reported having a stroke, a stroke was recorded in our medical records. Further, we did not collect information on neurological manifestations during previous acute episodes. Post hoc, we were able to document that 14 of the 31 Oklahoma Registry patients presented with severe neurological manifestations (ie, coma, weakness, stroke, partial numbness, or stroke symptoms) during at least 1 acute episode. It is unclear if the reported personality changes in this study were the result of dealing with residual symptoms or if the patients’ decreased desire to socialize was a result of their depression. The eligibility criteria of being in clinical remission was based solely on the length of time since the previous episode and did not require a normal ADAMTS13 activity value. However, remission ADAMTS13 activity levels have been collected for 30 of the Oklahoma Registry patients during our long-term follow-up. Most of them, 21 out of 30 (70%), had all normal ADAMTS13 activity remission levels, 8 out of 30 had a mixture of normal and deficient (<10% activity), and 1 patient had consistently deficient (<10% activity). Moreover, factors potentially influencing the patient experience, such as a history of drug or alcohol abuse, were not considered during recruitment. During the study, patients were instructed to list on the individual index cards their top 3 symptoms that affected their daily lives, and these instructions could have been interpreted differently by each patient (importance, frequency, severity, or interference of all 3). Finally, another limitation included that, because of the confidential nature of the focus group data, we were not able to link individual patient symptoms to demographic characteristics such as time in remission.
In conclusion, patients described that iTTP was a life-changing event and they often did not return to previous functioning but instead learned to function with life-changing residual impacts of the disease. Therefore, there is a necessity for a paradigm shift in the care of patients with iTTP from acute management to that of a multidisciplinary comprehensive care model. The best model of comprehensive care in rare blood disorders would include physicians, specialized nursing, physical therapy, social work, and pharmacy, to ensure full wrap-around care of the individual.27 The development of a tailored comprehensive care model for iTTP is essential to improve the care of these patients in remission. As a first step in that direction, it is important to begin to incorporate standardized assessments of these symptoms during remission using valid, reliable patient-reported outcome measures. Future prospective studies should focus on identifying if these residual symptoms are a result of surviving or treatments related to an iTTP diagnosis.
Acknowledgments
The authors thank the men and women who shared their personal experiences in our focus groups and interviews. The authors also thank James George for his assistance in the study and with the manuscript.
This project was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number 1K01HL135466 (D.R.T.), and Oklahoma Shared Clinical and Translational Resources NIH/NiGMS U54GM104938 (S.K.V.).
Authorship
Contribution: D.R.T. conceived and designed the study; M.K.C. was the qualitative methodology mentor; D.R.T., R.A.K., L.B.Q., A.J.L., and C.M.M. helped to recruit study participants; D.R.T. led focus groups and interviews; R.A.K., L.B.Q., C.M.M., and A.J.L. were assistant moderators and note takers in focus groups or interviews; D.R.T., R.A.K., and M.K.C. performed content analysis for focus groups and interviews; D.R.T. wrote the manuscript; R.A.K. created the visual abstract; and all authors reviewed the analysis and final manuscript.
J.A.P. contributed to this article while being an employee of the Medical College of Wisconsin. The views expressed are her own and do not necessarily represent the views of the National Institutes of Health or the US Government.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Deirdra R. Terrell, The University of Oklahoma Health Sciences Center, 801 NE 13th St, Hudson College of Health Building, Room 333, Oklahoma City, OK 73104; e-mail: dee-terrell@ouhsc.edu.
References
Author notes
Data are available on request from the corresponding author, Deirdra R. Terrell (dee-terrell@ouhsc.edu).