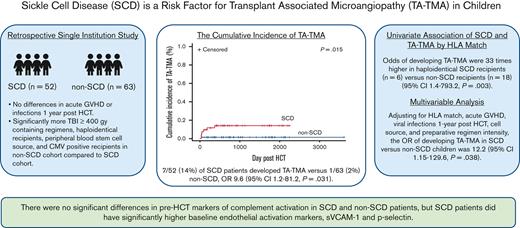
Key Points
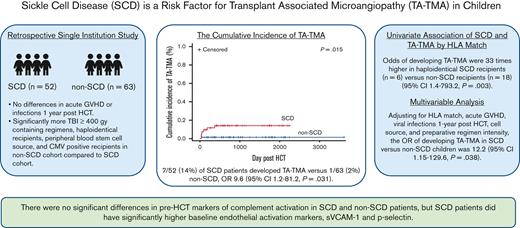
Children with SCD have a higher risk of developing TA-TMA even after adjusting for other features (OR, 12.22; 95% CI, 1.15-129.6; P = .038).
Pre-HCT complement markers were similar in children with and without SCD.
Abstract
Transplant-associated thrombotic microangiopathy (TA-TMA) and sickle cell disease (SCD) share features of endothelial and complement activation. Thus, we hypothesized that SCD is a risk factor for TA-TMA and that prehematopoietic cellular transplantation (HCT) markers of endothelial dysfunction and complement activation would be higher in patients with SCD. Children who underwent initial haploidentical or matched sibling donor HCT between January 2015 and June 2020 were included in this institutional review board–approved, single institution, retrospective study. Of the 115 children, 52 had SCD, and 63 underwent HCT for non-SCD indications. There was no significant difference in severe grade 3 to 4 acute graft-versus-host disease (GVHD) between recipients of HCT with or without SCD. The non-SCD cohort had significantly more cytomegalovirus-positive recipients, radiation-containing preparative regimens, and peripheral blood stem cell graft sources (P ≤ .05), all described risk factors for developing TA-TMA. Despite this, 7 of 52 patients (13%) with SCD developed TA-TMA compared with 1 of 63 patients (2%) without SCD (P = .015). Risk was highest in those who underwent haploidentical HCT (odds ratio [OR], 33; 95% confidence interval [CI], 1.4-793.2). Adjusting for HLA match, GVHD, post-HCT viral infection, stem cell source, and myeloablation, SCD remained a risk for developing TA-TMA (OR, 12.22; 95% CI, 1.15-129.6). In available pre-HCT samples, there was no difference in complement biomarkers between those with SCD and those without, though patients with SCD did have significantly higher levels of markers of endothelial activation, soluble vascular cell adhesion molecule 1, and P-selectin. In conclusion, children with SCD merit careful screening for TA-TMA after HCT, particularly those receiving a haploidentical HCT.
Introduction
Sickle cell disease (SCD), the most common inherited blood disorder in the United States, is characterized by recurrent, debilitating pain crises, organ dysfunction, and a shortened life span.1,2 A single amino acid substitution results in polymerization of the sickle hemoglobin, resulting in chronic hemolysis under conditions of hypoxic stress. Intravascular hemolysis and mechanical interactions of deformed red blood cells with the endothelial lining of the blood vessel result in endothelial activation.3-7 Free heme and endothelial activation may lead to complement activation in SCD, and there are several reports of complement-mediated thrombotic microangiopathy (TMA) in patients with SCD who have not undergone transplantation in the setting of vaso-occlusion and hyperhemolysis.8,9 Currently, treatment options for SCD include medication interventions (eg, hydroxyurea), investigational genetic therapy approaches, and hematopoietic cellular transplantation (HCT), which is the only readily available curative option with long-term data.
Endothelial and complement activation are also central mechanisms of transplant-associated TMA (TA-TMA), a common and potentially severe complication of HCT.10-12 Microthrombi formation in the vasculature results in multiorgan dysfunction and/or death in approximately half of the children with severe TA-TMA.13 Described risk factors for TA-TMA include myeloablative conditioning regimens, particularly with total body irradiation, HLA mismatch, acute graft-versus-host disease (GVHD), infections, and variations in complement genes.14-17 Prolonged endothelial activation can result in endothelial dysfunction, which is thought to predispose patients to TA-TMA.14 However, there is scant literature exploring whether differences in serum measurements of endothelial activation before HCT are associated with later development of TA-TMA.
Aside from underlying endothelial activation, children with SCD undergoing HCT typically have few previously described TA-TMA risk factors. Outside of a clinical trial, the standard of care is to proceed with HCT only when young recipients have an available, young, matched sibling donor (MSD). Although the incidence of TA-TMA in children after allogeneic HCT is thought to be 20% to 30%, the incidence in those with SCD is unknown.18 To date, only a few case reports of TA-TMA in recipients of HCT with SCD are published.19,20
We hypothesized that recipients with SCD are at increased risk for developing TA-TMA as a result of years of underlying endothelial activation and baseline-activated complement.3,6 Identifying whether SCD confers an increased risk for TA-TMA after HCT could better inform patients and caregivers and potentially permit preemptive or preventive strategies in those at highest risk. The objective of this study was to determine the association between developing TA-TMA in recipients of HCT with SCD and whether there are measurable differences in markers of endothelial and complement activation before HCT in patients with and without SCD.
Methods
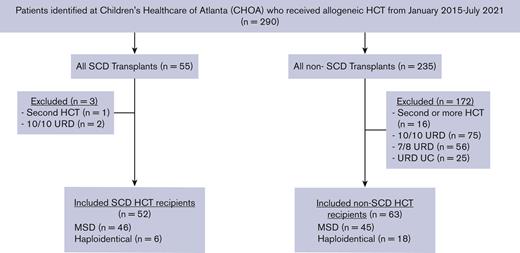
In this institutional review board–approved, retrospective, cohort study, consecutive patients who underwent a first allogeneic stem cell transplant with an MSD or haploidentical donor between January 2017 and July 2021 at Children’s Healthcare of Atlanta were identified using an internal database (Figure 1). Patients receiving their second or greater allogeneic HCT were excluded, as this is a described risk factor for TA-TMA15,17 and 10 out of 10 patients receiving transplants from URDs were excluded based on the very small number in the SCD cohort.
Patient selection schema. All MSD and haploidentical first allogeneic HCTs were included in the study. UC, umbilical cord; URD, unrelated donor.
Patient selection schema. All MSD and haploidentical first allogeneic HCTs were included in the study. UC, umbilical cord; URD, unrelated donor.
Transplant and clinical characteristics were extracted from the medical chart of all eligible patients.
Definitions
TA-TMA was diagnosed using the following criteria from Jodele et al, including ≥4 of the following: de novo anemia or increased packed red blood cell transfusion requirements, de novo thrombocytopenia or increased platelet transfusion needs, elevated lactate dehydrogenase, presence of schistocytes, proteinuria (≥30 ng/mL), hypertension ≥99th percentile, or in those aged ≥18 years, blood pressure ≥140/90 mm Hg, or elevated soluble terminal complement complex 9 (sC5b-9) (≥ upper limit of normal).10,21 All patients had TA-TMA criteria applied retrospectively for the first year after first HCT. TA-TMA diagnosis was made either by extraction of the diagnosis from the chart or by retroactive diagnosis. Veno-occlusive disease (VOD) was defined using Baltimore criteria.22 TA-TMA and VOD diagnoses were adjudicated by 3 separate authors with 100% agreement required. Severe acute GVHD was staged and graded using Mount Sinai Acute GVHD International Consortium criteria.23 Preparative regimen intensity was defined using Center for International Blood and Marrow Transplant Research definitions as myeloablative vs reduced intensity. Nonrelapse mortality (NRM) was defined as death not related to relapse. Infections within the first year after HCT were detected by cultures for bacteremia and by polymerase chain reaction for viral infections. Fungal infections were identified with either culture, next-generation sequencing or fungal markers, and consistent imaging for fungal disease.
Statistical analysis
The objectives of this study were to determine the odds of developing TA-TMA in patients with SCD vs contemporaneous patients without SCD and to determine whether there are differences in markers of endothelial or complement activation before HCT. Patient and transplant characteristics are reported descriptively and compared among groups using χ2, Fischer exact, or Wilcoxon rank-sum test as appropriate. Overall survival was estimated using a Kaplan-Meier curve, and groups were compared using a log-rank test. The cumulative incidence of TA-TMA in patients with and without SCD was calculated and compared using the Gray test. In a univariate analysis, odds ratios (ORs), risk ratios (RRs), relative risk differences (RDs), and 95% confidence intervals (CIs) were calculated to measure the association between TA-TMA and SCD. Logistic regression was used to determine the adjusted association between SCD and TA-TMA. The model was selected a priori, based on the known risk factors for TA-TMA. In addition, a sensitivity analysis including multiple different models was performed to determine the robustness of the adjusted association of SCD and TA-TMA.24 Exploratory analyses included determining the RR and OR of developing TA-TMA within the MSD and haploidentical groups and determining whether there were significant differences in pre-HCT endothelial or complement markers in patients with and without TA-TMA.
Baseline markers of complement and endothelial activation were normally distributed; thus, patients with and without SCD were compared using t tests. Correlations between complement and endothelial markers were tested using Spearman correlation coefficient. Significance level was set at P < .05, and statistical testing was performed using SAS version 9.4.
Endothelial and complement markers
All patients with an available pre-HCT sample in an institutional biorepository (SCD = 22, non-SCD = 17, total = 39) had biomarkers tested. Per laboratory standard operating procedures, samples were processed within 2 hours of being received and stored in a −80°C freezer. Complement activation markers (sC5b-9 [Quidel #A020], C5a [Quidel # A021], C3a [Quidel #A031], and Bb [Quidel #A027]) were measured in duplicate using enzyme-linked immunosorbent assay kits per the manufacturer’s instructions. Markers of endothelial activation were measured in duplicate using customized Meso Scale Discovery Multiplex R-PLEX kits per the manufacturer’s instructions. Endothelial markers measured included syndecan-1, suppression of tumorgenicity 2 protein (ST-2), P-selectin, soluble vascular cell adhesion molecule 1 (sVCAM-1), and soluble intracellular adhesion molecule 1 (ICAM-1).
Results
One hundred fifteen patients who underwent allogeneic HCT were included in the study, 52 with SCD and 63 with non-SCD indications. There was no significant difference in mean age; sex; incidence of grade 3 to 4 severe acute GVHD; or bacterial, viral, or fungal infections 1 year after HCT between patients with and without SCD. The non-SCD cohort had significantly more cytomegalovirus-positive recipients (76% vs 38%, P < .0001), more radiation-containing preparative regimens (P < .0001), and more peripheral blood stem cell (PBSC) graft sources (P = .0008); all are described risk factors for developing TA-TMA. There were significantly more Black patients (P < .001) and more myeloablative preparative regimens (P = .05; Table 1) in the SCD cohort. Standard TA-TMA screening was implemented in 2019; 58 (50%) patients received transplants before the implementation of screening and 57 (50%) after screening. Features of patients in pre- and postscreening implementation are summarized in supplemental Table 1.
SCD
Among the recipients of HCT with SCD, 11 patients had an abnormal transcranial doppler, 4 had strokes, and 14 required chronic packed red blood cell transfusions. Most patients were able to manage vaso-occlusive pain at home, although 10 (19%) required 3 or more hospitalizations for the treatment of vaso-occlusive crises (VOCs) the year before undergoing HCT (Table 2). Thirty-nine patients (75%) with SCD were on hydroxyurea before undergoing HCT. Seven (13.5%) developed TA-TMA, a median of day 71 after HCT (range, 30-546 days). Six patients were diagnosed with TMA in real time, and 1 was retrospectively identified. Of the 6 haploidentical HCT recipients, 3 (50%) developed TA-TMA. More patients with TA-TMA developed posterior reversible encephalopathy syndrome than those without TA-TMA (29% vs 2%; P = .04; Table 2). Three patients were treated with eculizumab (a complement C5 inhibitor), 3 required changes in immune suppression medications, and 1 was observed with resolution over 3 months. Four had severe grade 3 to 4 acute GVHD concurrent with TA-TMA. All patients are currently alive with a median follow-up of 747 days (range, 67-1587 days) (supplemental Table 2).
Non-SCD
Of those who underwent HCT for non-SCD indications (n = 63), 28 had nonmalignant hematologic disorders, and the remaining 35 had a hematologic malignancy (Table 1). One patient (2%) with concurrent stage 4, grade 4 lower gut acute GVHD and BK hemorrhagic cystitis was diagnosed with TA-TMA on day 34. He was treated with eculizumab but had no improvement in hematologic or organ dysfunction and died on day 134. No patient retrospectively met criteria for TA-TMA. Thirteen (37%) patients with a malignancy relapsed, and 49 (78%) were alive at the last follow-up (median, 960 days; range, 156 days to 9.9 years). Three children died of NRM, including 1 with severe VOD in the setting of engraftment failure and infection and 1 with TA-TMA with concurrent severe GVHD and diffuse alveolar hemorrhage.
Risk of TA-TMA in SCD vs non-SCD
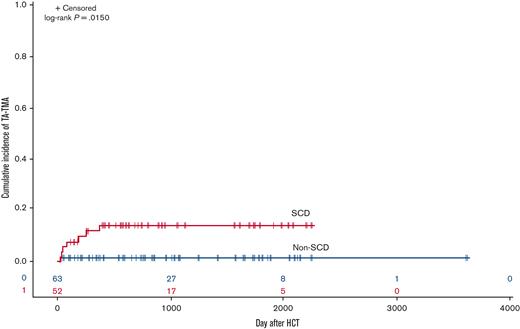
The estimated cumulative incidence of developing TA-TMA in the SCD cohort vs non-SCD cohort on day 100 was 8% vs 2% and 1 year after HCT was 12% vs 2%, respectively (P = .015; Figure 2). The odds of developing TA-TMA in patients with SCD were 9.6-fold higher (95% CI, 1.2-81.2; P = .031) than in patients without SCD, with a RD of 11.8% (95% CI, 2.1%-21.7%). When comparing the odds of TA-TMA within donor source, recipients of haploidentical HCT with SCD had the highest risk of developing TA-TMA with an OR of 33 (95% CI, 1.4-793.2; P = .003) compared with patients without SCD. OR, RR, and RDs with 95% CI are shown in Table 3.
Cumulative incidence of TA-TMA in SCD vs non-SCD cohorts. Patients with SCD had a significantly higher cumulative incidence of TA-TMA than the non-SCD cohort (P = .0150).
Cumulative incidence of TA-TMA in SCD vs non-SCD cohorts. Patients with SCD had a significantly higher cumulative incidence of TA-TMA than the non-SCD cohort (P = .0150).
In a multivariable model adjusting for HLA match (8/8, haploidentical), acute GVHD (grade 0, grade 3-4), viral infection (yes, no), stem cell source (bone marrow, PBSC, or UC) and myeloablative preparative regimen (yes, no), SCD remained significantly associated with the development of TA-TMA (OR, 12.22; 95% CI, 1.15-129.6; P = .038; Table 4). In a sensitivity analysis, we estimate that the adjusted OR of developing TA-TMA in patients with SCD ranges from 9.03 to 21.12 (supplemental Table 3).
Pre-HCT complement and endothelial activation markers
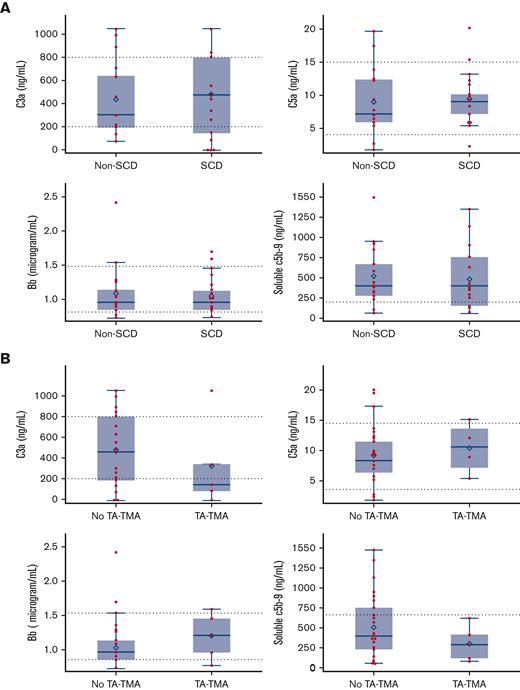
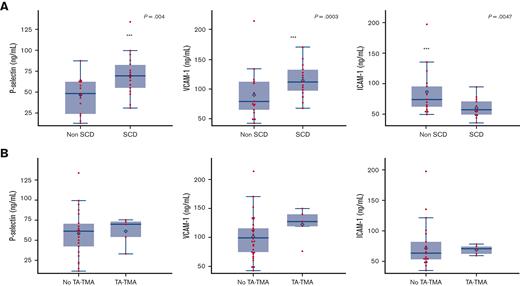
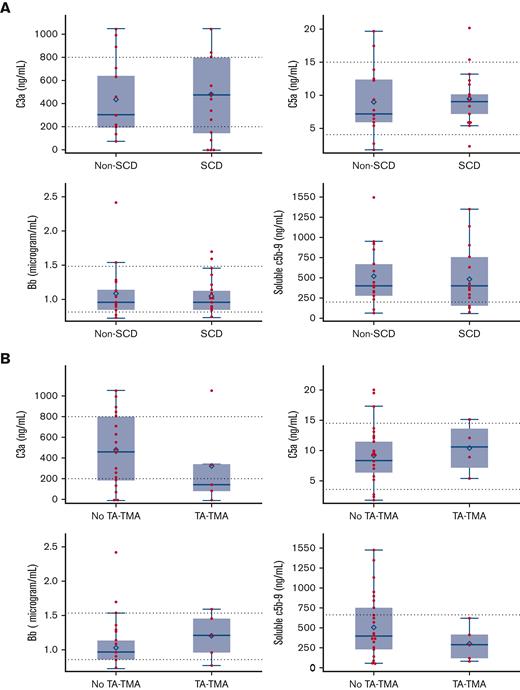
No patients with SCD had a VOC at the time samples were collected. Features of patients with available samples compared with those without available samples are summarized in supplemental Table 4. Complement pathway activation was investigated by examining the alternative pathway (Bb), anaphylatoxins (C3a and C5a), and sC5b-9. There was no statistical difference in pre-HCT sC5b-9, C5a, C3a, and Bb in patients with or without SCD (Figure 3A) or in patients with or without TA-TMA (Figure 3B). Both patients with and without SCD had baseline complement values largely within the normal range, except for sC5b-9, which was elevated in both groups. Among the markers of endothelial activation tested before HCT, children with SCD had significantly higher mean P-selectin (SCD, 68.80 ng/mL; standard deviation [SD], 23.57 vs without SCD, 45.58 ng/mL; SD, 21.22; P = .0027) and sVCAM-1 (SCD, 1146.606 ng/dL; SD, 261.99 vs without SCD 903.53 ng/dL; SD, 417.68; P = .03) than those without SCD (Figure 4A). There was no significant difference seen in ST-2, syndecan-1, and endothelin-1 levels (data not shown). E-selectin levels were significantly higher in the non-SCD cohort than in the SCD cohort (857.36 ng/dL; SD, 140.20 vs 597.71 ng/dL; SD, 140.20, respectively; P = .0047) (data not shown). There were no differences in baseline endothelial markers between patients who developed TA-TMA and those who did not (Figure 4B). There was no significant correlation between any complement markers and endothelial markers.
Pre-HCT complement activation markers in patients with and without SCD and in those who later did or did not develop TA-TMA. (A) Among patients with an available baseline pre-HCT sample (SCD = 22, non-SCD = 17, total = 39), there was no significant difference in pre-HCT levels of complement markers sC5b-9, C3a, C5a, or Bb in either the SCD or non-SCD cohorts. (B) There was no difference between those who later did develop TA-TMA (samples available, n = 7, all patients with SCD) and those who did not develop TA-TMA (n = 31). Dashed lines indicate upper and lower limits of normal. Box and whisker plots indicate the median and quartiles. Each value is represented by a red dot and the mean by a blue diamond.
Pre-HCT complement activation markers in patients with and without SCD and in those who later did or did not develop TA-TMA. (A) Among patients with an available baseline pre-HCT sample (SCD = 22, non-SCD = 17, total = 39), there was no significant difference in pre-HCT levels of complement markers sC5b-9, C3a, C5a, or Bb in either the SCD or non-SCD cohorts. (B) There was no difference between those who later did develop TA-TMA (samples available, n = 7, all patients with SCD) and those who did not develop TA-TMA (n = 31). Dashed lines indicate upper and lower limits of normal. Box and whisker plots indicate the median and quartiles. Each value is represented by a red dot and the mean by a blue diamond.
Pre-HCT markers of endothelial activation in patients with SCD vs patients without SCD and those who later did or did not develop TA-TMA. (A) Among patients with an available baseline pre-HCT sample (SCD = 22, non-SCD = 17, total = 39), P-selectin and VCAM-1 were significantly higher in children with SCD than in those without SCD. There was no difference in syndecan-1, ST-2, or endothelin-1 in the SCD vs non-SCD cohorts (not shown). (B) There was no difference in pre-HCT levels of endothelial markers in those who later developed TA-TMA (samples available, n = 7, all patients with SCD) vs those who did not develop TA-TMA (n = 31). Box and whisker plots indicate the median and quartiles. Each value is represented by a red dot and the mean by a blue diamond.
Pre-HCT markers of endothelial activation in patients with SCD vs patients without SCD and those who later did or did not develop TA-TMA. (A) Among patients with an available baseline pre-HCT sample (SCD = 22, non-SCD = 17, total = 39), P-selectin and VCAM-1 were significantly higher in children with SCD than in those without SCD. There was no difference in syndecan-1, ST-2, or endothelin-1 in the SCD vs non-SCD cohorts (not shown). (B) There was no difference in pre-HCT levels of endothelial markers in those who later developed TA-TMA (samples available, n = 7, all patients with SCD) vs those who did not develop TA-TMA (n = 31). Box and whisker plots indicate the median and quartiles. Each value is represented by a red dot and the mean by a blue diamond.
Discussion
In this single-center, pediatric, retrospective study, recipients of HCT with SCD had significantly higher odds of developing TA-TMA than recipients of HCT without SCD after adjusting for HLA match, acute GVHD, viral infections, cell source, and preparative intensity (OR, 12.2; 95% CI, 1.15-129.6; P = .038). The odds of developing TA-TMA were highest in recipients of haploidentical HCT with SCD; however, this was a small cohort. The recipients of haploidentical HCT, who developed TA-TMA had severe SCD before undergoing HCT with frequent VOC and developed concurrent grade 3 to 4 acute GVHD. The haploidentical trial (clinicaltrials.gov #NCT02757885 ) continues to accrue patients, although mycophenolate was added for GVHD prophylaxis after an interim analysis. The preparative regimen these patients received included hydroxyurea on days −100 to −10, fludarabine (total 150 mg/m2), rabbit antithymocyte globulin (total 4.5 mg/kg), thiotepa (10 mg/kg), cyclophosphamide (29 mg/kg), and total body irradiation (2 Gy) with acute GVHD prophylaxis of posttransplant cyclophosphamide and sirolimus. Of note, other haploidentical clinical trials in SCD have not reported TA-TMA as a complication,25 but many centers do not perform routine TA-TMA screening, and the diagnosis is not routinely captured in clinical trials. It is unclear whether a high incidence of TA-TMA in this small haploidentical cohort is related to concurrent GVHD, the transplant approach, underlying SCD itself, or a combination of these risks.
TA-TMA is a clinical diagnosis. Although multiple criteria are proposed, they all rely on a combination of nonspecific criteria. In the SCD setting, patients are at increased risk for abnormalities in lactate dehydrogenase levels, urine protein-to-creatinine ratios, pulmonary hypertension, and development of posterior reversible encephalopathy syndrome, a complication seen in TA-TMA. Further, patients with SCD often have higher hemoglobin and platelet transfusion parameters, making it more challenging to interpret transfusion needs. It is unknown if or how this affects the application of TA-TMA clinical criteria in this population. Geographic and genetic ancestry are not known as independent risk factors for the development of TA-TMA; however, they are risk factors for the development of many other diseases. In a study analyzing variants of complement genes to determine genetic susceptibility of TA-TMA, non-White children were more likely to have more complement gene changes, and an increased number of complement genetic variants was associated with increased NRM.26,27 The authors hypothesized that patients of sub-Saharan African descent may have more activating variants in complement genes, conferring a protective advantage against malaria, but perhaps increasing the risk for TA-TMA. Deletions in complement factor H-related genes 3 and 1 are described in TA-TMA and are highly variable by geography; deleted allele frequency in 1 study ranged from 54.7% in individuals of Nigerian ancestry to 0% in individuals of Japanese and South American ancestry.28 Given the long-standing endothelial activation in SCD, we hypothesized that SCD is an independent risk for TA-TMA outside of ancestry but did not have the sample size in this study to confirm this. Genetic predisposition may have implications in the risk of developing TA-TMA, particularly in patients of African ancestry who have SCD, although larger studies are needed to explore these implications.
There is emerging evidence that complement is activated at baseline in SCD,3,29 and complement activation is known to be a key driver of TA-TMA.21 We hypothesized that patients with SCD would have increased activation of complement before HCT and that this could be associated with increased risk of TA-TMA. However, we were unable to detect any significant differences in baseline complement activation in SCD and non-SCD cohorts. Similarly, we did not detect any differences before HCT between the patients who later developed TA-TMA and those who did not. There are several possible nuances to this observation. The children in this cohort were very different from the adults, with severe and largely untreated SCD, in which complement activation has been previously tested.3 In our cohort, most patients had mild disease and received a preemptive transplant, and organ function was excellent; all patients had either simple or exchange packed red blood cell transfusions to reduce hemoglobin S to less than 30% before HCT when samples were drawn, and no patient had a VOC at the time the samples were obtained. Furthermore, 39 patients (75%) were on hydroxyurea before HCT, which is known to lead to substantial reduction in organ dysfunction and complement activation.3 Any or all of these features could have affected baseline sC5b-9 levels. In addition, a recent report from our group showed that measurements of complement markers in SCD during vaso-occlusive pain crisis were significantly higher but in the absence of a VOC were close to normal.9 Thus, children whose SCD is managed with optimal preventive care may not have differences at baseline, but they may have differences in complement activation when exposed to stressors such as chemotherapy. This would not be captured in our study. Serial measurements of complement activation levels, including at the time of TA-TMA diagnosis would be insightful; unfortunately, these samples were not available in our cohort.
Some studies suggest that early (28 days after HCT) elevated sC5b-9 may be associated with subsequent development of TA-TMA,22 but baseline sC5b-9 levels have not been described as a known predictor for TA-TMA development. In this study, pre-HCT sC5b-9 levels varied widely among both SCD and non-SCD cohorts, and there was no association with baseline measurements and the development of TA-TMA. Most studies investigating complement markers through the HCT process are relatively small and include a heterogenous group of patients, limiting the interpretation of these data. Although differences in genetic variations of complement by ancestry are described,28 it is unknown if this also affects the serum complement markers. To further determine the implications of sC5b-9 levels at baseline and at the time of TA-TMA diagnosis, particularly in SCD, additional studies are needed to determine if there are differences in normal ranges by ancestry and to characterize ranges of normal values in the posttransplant setting.
A “3 hit hypothesis” for TA-TMA development has been proposed, with the first hit including complement activation or endothelial dysfunction.14 However, little is known about the predictive value of baseline endothelial dysfunction and later development of TA-TMA. There are some data indicating that markers of endothelial activation are detectably different at the time of TA-TMA diagnosis and other HCT complications,30 but there are few studies that have investigated whether differences in endothelial markers before HCT are associated with later TA-TMA development. Given the known endothelial activation and dysfunction in SCD, we hypothesized that patients with SCD would have significant differences in markers of endothelial health, even when compared with patients without SCD, including those with pretreated leukemia. We found that patients with SCD had significantly elevated sVCAM-1 and P-selectin, both of which are nonspecific markers of endothelial activation, which could support the first hit hypothesis. There were no significant differences in other endothelial markers except, curiously, elevated ICAM-1 in the non-SCD cohort. The etiology of this difference is unclear, although some data suggest that E-selectins are elevated in patients with hematologic malignancies.31 There was also no difference in baseline endothelial markers of patients who later developed TA-TMA, although the small number of patients may have precluded the ability to discern differences.
Of note, the incidence of TA-TMA in the non-SCD cohort is much lower than that reported in other cohorts, with an estimated cumulative incidence of 2% at 1 year compared with most studies, which report an incidence of 20% to 40%.10,15,18 In part, we anticipated this in our cohort enriched for young patients with few TA-TMA risk factors, largely MSD and a low incidence of severe GVHD. In addition, routine TA-TMA screening was not in place at our institution until 2019; although awareness of TA-TMA increased over time, lack of screening laboratories for all patients is a limitation to this study. We attempted to overcome this limitation by retrospectively applying TA-TMA criteria to the entire cohort. However, screening labs were sent at the discretion of the treating physician. It is likely that we identified only the most severe cases of TA-TMA in this study. Although the incidence of TA-TMA may be underestimated, we would expect this effect to be similar across SCD and non-SCD cohorts. Thus, both groups should be similarly affected by screening practice, suggesting that this is unlikely to affect our results.
Although this is a relatively large number of patients for a pediatric SCD HCT study, small numbers remain a limitation. Selection of an appropriate non-SCD cohort was challenging; we opted to include all non-SCD indications but would have preferred to exclude those indications which may have increased risk for TA-TMA; for example, Fanconi anemia and severe aplastic anemia.32 However, to improve the power of our study, we elected to include all patients and to only exclude second transplant recipients, for which there is robust data showing increased TA-TMA risk.15,17 An added limitation of our study was the inclusion of only recipients of haploidentical and MSD HCT, which was chosen as these donors constituted most institutional protocols and practice for patients with SCD receiving HCT during the period evaluated by our study. A final limitation to our data is that the results of complement markers can be affected by processing. This risk is mitigated by the fact that all samples were collected on a single institutional biorepository following a standard operating procedure. Sample numbers were limited owing to the patients who consented to this study.
Collectively, our data support that patients with SCD have a higher risk of TA-TMA, and those undergoing haploidentical transplantation may be at highest risk. We promote that all patients after HCT be carefully monitored for TA-TMA; however, patients with SCD should be given particular attention, especially those with unrelated or mismatched donor approaches, who are known to have additional risk factors for TA-TMA, including GVHD.33 Patients with SCD may have differences in endothelial activation before HCT compared with patients without SCD, even patients with heavily pretreated for leukemia, with an elevated sVCAM-1 and P-selectin before HCT. If validated in a larger prospective study, these data could be integral to identify patients at higher risk of morbidity and mortality from HCT, permitting individual risk stratification and identification of patients who would most benefit from future TA-TMA preemptive or prophylactic strategies.
Acknowledgments
The authors acknowledge Matthew Magee, biostatistician, for reviewing the analysis.
This work was supported by funding from the National Institutes of Health Loan Repayment Program, the Aflac Pilot Award (M.S.), and the American Society of Hematology Scholar Award (S.C.).
Authorship
Contribution: A.H. extracted data; M.S. designed the study, completed the analysis, and wrote the manuscript; S.C. and J.J. completed the complement marker analysis; S.C. and K.M.W. helped with study design and edited the manuscript; K.S., D.L., and S.R. extracted data from the medical record and helped edit the manuscript; E.S., S.P., M.Q., B.W., and K.L. edited the manuscript and cared for the patients; K.S., D.L., and S.R. manually extracted information from the medical chart, with other authors participating in the adjudication of 25% of randomly selected data in addition to TA-TMA and VOD diagnoses; and all authors performed chart audits to verify retrospective assignment of TA-TMA.
Conflict-of-interest disclosure: S.C. has provided consultation to Agios, Alexion, Daichi Sankyo, Novartis, and Takeda and has received research funding from Alexion and Global Blood Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Michelle Schoettler, Children's Healthcare of Atlanta/Aflac Cancer and Blood Disorders Center, 1405 Clifton Rd, NE, Atlanta, GA 30322; e-mail: Michelle.Schoettler@emory.edu.
References
Author notes
∗K.M.W. and S.C. are joint senior authors.
Data are available on request from the corresponding author, Michelle Schoettler (michelle.schoettler@emory.edu).
The full-text version of this article contains a data supplement.