TO THE EDITOR:
Sickle cell disease (SCD) describes any of several related genetic disorders that affect nearly 100 000 individuals in the United States and are associated with significant morbidity and mortality.1 The most common complication of SCD, a painful vasoocclusive event (VOE), is typically managed in the home with heat, relaxation, and hydration, frequently combined with oral opioid and nonopioid adjuvants.2
Patients with VOEs who are unresponsive to home-care pain-management strategies frequently require urgent medical evaluation and treatment with IV opioids delivered at high doses or for prolonged intervals. Guidelines recently published by the American Society of Hematology recommend repeated IV analgesics beginning within 1 hour of patient presentation.3 In practice, patients who present for emergency department (ED) care are frequently met by long delays until the first administration of analgesic, resulting in rapid progression of the VOE condition and an increased likelihood of inpatient admission. In contrast, patients who are evaluated and managed in hematology/oncology outpatient infusion centers more commonly receive guideline-concordant care with a concomitant reduction in hospital admission.4-10 Although the benefit of rapid analgesia in this setting is clear, there is little evidence that directly speaks to other determinants of successful pain management: specifically, the frequency and quantity of second and subsequent opioid doses. There is a critical need for evidence that supports specific pharmacologic interventions for the treatment of painful VOE, with the goal of improving patient response, reducing encounter duration, and decreasing the incidence of therapeutic failures that can lead to ED and/or inpatient admissions.
To streamline patient care and to establish evidence-based pain management strategies for treating acute sickle cell VOEs, we implemented an interdisciplinary collaboration between the Penn Comprehensive Sickle Cell Program (CSCP) and the Hematology-Oncology Evaluation Center (OEC) at the Hospital of the University of Pennsylvania. The OEC is a same-day outpatient infusion clinic where advanced practice providers evaluate and manage patients for symptoms related to their specific hematology/oncology conditions. As part of this mission, the OEC cares for patients with sickle cell VOEs who are referred by their Penn CSCP providers. The present study reports early data on a novel treatment paradigm that improves the efficiency of patient care without sacrificing therapeutic effectiveness. Specifically, we hypothesized that administration of IV opioids at 15-minute intervals (short interval), rather than the customary 60-minute intervals (standard interval), would provide adequate analgesia, reduce the total dose of opioids administered, and shorten the duration of the OEC encounter.
All patients in this observational study received their primary SCD care within the Penn CSCP and were treated for their acute, uncomplicated VOEs in the OEC at the Perelman Center for Advanced Medicine. The study population comprised an unselected group of 16 patients, all of whom had SCD (Table 1). All patients were treated in the OEC, 7 who received both standard- and short-interval treatment and 9 who received short-interval treatment only (Table 1). Data and metrics for this study were collected over an 18-month interval between March 2020 and August 2021, for a total of 67 visits. Patients treated in the 10 months preceding 1 January 2021 received standard-interval treatment, whereas those treated in the 8 months after this date received short-interval treatment. Specific per-patient details, including sickle cell genotype, number of infusion center visits before and after intervention, and home opioid plan doses (as milligram morphine equivalents [MME]) are listed in Table 1. All IV opioids were administered by IV push over 1 to 2 minutes. Each patient’s infusion center opioid dose was preestablished by MME correlation and was not titrated during the visit, but dose could be altered at subsequent visits should pain not be relieved by previous opioid doses. A maximum of 3 doses of IV opioids were allowed regardless of pain level, and patients were monitored for 30 to 60 minutes after the final dose of IV opioid to ensure clinical stability and resolution of symptoms. If the patient’s pain was not at a manageable level after the third dose of IV opioid, we referred the patient to be evaluated in the Penn Medicine Emergency Department. Inclusion and exclusion criteria, treatment guidelines, and discharge criteria for treatment in the Penn Hematology-OEC are listed in supplemental Table 1. This project was reviewed and determined to be institutional review board–exempt by the University of Pennsylvania’s Institutional Review Board, as it was determined to be a quality improvement; it was conducted according to the Declaration of Helsinki.
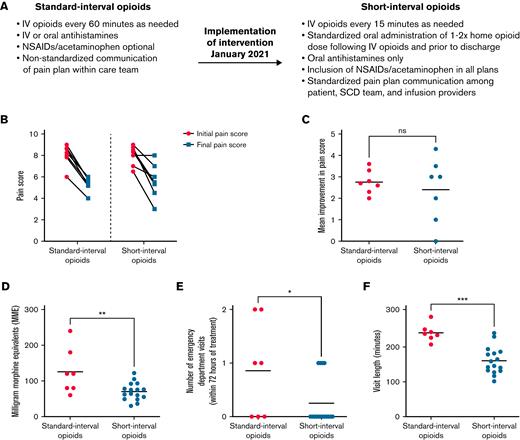
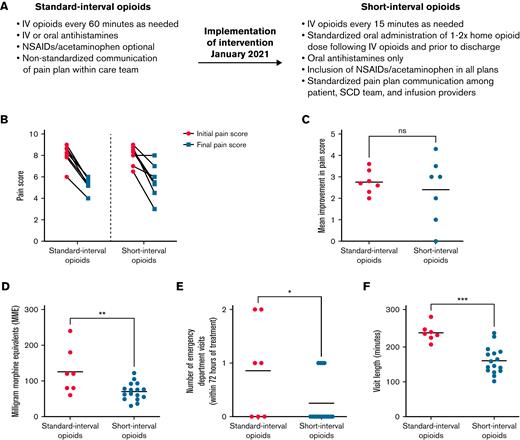
The genesis of this study arose from members of the interdisciplinary treatment team who recognized the need for evidence-based pain management regimens for treating patients with sickle cell VOEs. Historically, patients with acute pain episodes that required IV analgesia could spend up to 6 hours in the OEC. Standard pain management plans included nonsteroidal anti-inflammatory drugs (NSAIDs) and any of several opioid medications delivered every 60 minutes, depending on the provider’s choice and the patient’s response. Although current SCD guidelines do not advocate for 60-minute intervals between opioid doses,1,3 this longer interval between opioids had been our previous institutional practice, and very few prior studies have specifically studied the role of shorter opioid intervals on patient outcomes. Thus, we hypothesized that SCD vaso-occlusive episodes could be treated effectively with lower total doses of opioids if delivered at shorter intervals that corresponded to the pharmacological peak of drug activity. For each patient, the IV opioid dose was based on the oral MME dose specified on the patient’s home pain plan. In contrast, the interval for repeat IV opioid administration was the same for all patients, based on pharmacological principles for each drug (onset of action, peak action, and duration of activity). This consideration reduced the interval between opioid doses to every 15 minutes from the interval of 60 minutes, which had been the previous default standard (Figure 1A). To further standardize pain plans, to reduce posttreatment sedation, and to ensure safe discharge, we restricted antihistamines to oral form only. Additional key modifications to the short-interval treatment plans included standardized oral administration 1 to 2 times of the patient’s home opioid dose after IV opioids and before discharge; standardized administration of adjuvants, including nonsteroidal anti-inflammatory drugs and acetaminophen; and timely communication by the primary hematologist of changes to home pain plans (Figure 1A). Specific details regarding standard-interval vs short-interval pain plans are provided in a deidentified manner in the supplemental Appendix.
Short-interval opioid treatment using an optimized infusion center pain-management plan leads to equivalent reduction in patient-reported pain scores with significantly improved opioid usage, fewer ED referrals, and decreased visit length. (A) Details of standard-interval vs short-interval opioid treatment plans. (B) Patient-reported pain scores upon presentation and OEC discharge for patients receiving both standard- and short-interval therapy. Pain is reported on a scale from 1 (minimal pain) to 10 (maximal pain). (C) Mean improvement in pain score for patients receiving both standard- and short-interval therapy. P = NS by Wilcoxon matched-pairs signed rank test. (D) Total opioids administered (IV plus oral opioids, measured as MME) in patients receiving standard- and short-interval treatments. ∗∗P < .01 by Student t test. (E) Outpatient treatment failures in patients receiving standard- or short-interval treatment as measured by the number of ED visits within 72 hours of discharge from the infusion visit. ∗∗∗P < .001 by Student t test. (F) Total length of outpatient infusion visits for patients receiving standard- or short-interval treatment. ∗P < .05 by Student t test. NS, not significant; NSAID, nonsteroidal anti-inflammatory drug; SCD, sickle cell disease.
Short-interval opioid treatment using an optimized infusion center pain-management plan leads to equivalent reduction in patient-reported pain scores with significantly improved opioid usage, fewer ED referrals, and decreased visit length. (A) Details of standard-interval vs short-interval opioid treatment plans. (B) Patient-reported pain scores upon presentation and OEC discharge for patients receiving both standard- and short-interval therapy. Pain is reported on a scale from 1 (minimal pain) to 10 (maximal pain). (C) Mean improvement in pain score for patients receiving both standard- and short-interval therapy. P = NS by Wilcoxon matched-pairs signed rank test. (D) Total opioids administered (IV plus oral opioids, measured as MME) in patients receiving standard- and short-interval treatments. ∗∗P < .01 by Student t test. (E) Outpatient treatment failures in patients receiving standard- or short-interval treatment as measured by the number of ED visits within 72 hours of discharge from the infusion visit. ∗∗∗P < .001 by Student t test. (F) Total length of outpatient infusion visits for patients receiving standard- or short-interval treatment. ∗P < .05 by Student t test. NS, not significant; NSAID, nonsteroidal anti-inflammatory drug; SCD, sickle cell disease.
To test the hypothesis that lower doses of IV opioids, when administered every 15 minutes, would both provide adequate analgesia and reduce the total opioid administered, we first assessed the effectiveness of the short-interval pain strategy using patient-reported pain scores (Figure 1B-C). The 7 patients who received both standard- and short-interval therapy reported improvements in pain scores at each visit (Figure 1B-C); importantly, the clinical benefits were equivalent regardless of opioid timing (standard-interval care, 8.1-5.3; short-interval care, 8.0-5.6) and were not dependent on time to first opioid, which remained unchanged between the 2 study groups (mean time to first opioid: 34 minutes in the standard-interval group vs 37 minutes in the short-interval group). This result indicates that patient satisfaction, measured as patient-reported pain score, can be maintained when IV opioids are delivered at an interval that is based on pharmacological principles.
To assess the effects of standard- and short-interval therapies on other outcomes, we compared 3 additional metrics total opioid dose, visit length, and number of treatment failures requiring a subsequent ED encounter in 16 patients who received either 1 or both treatment regimens. Patients who were given short-interval therapy received 45% less opioid (IV plus oral opioids, measured as MME) than those who received standard-interval therapy (Figure 1D). This overall decrease in opioid dose did not adversely affect VOE relapse in the 72 hours after OEC discharge; in fact, the average number of ED encounters was reduced by more than twofold after short-interval treatment (Figure 1E). Importantly, return ED visits within 72 hours did not directly correlate with total MME (data not shown), suggesting that short-interval therapy did not inadvertently lead to increased opioid withdrawal. Finally, our data indicate that short-interval therapy is resource efficient, with the total length of the outpatient infusion visit reduced by more than one-third compared with standard-interval therapy (Figure 1F; 235 minutes vs 157 minutes). These data demonstrate that short-interval therapy improves the efficiency of OEC workflow while both reducing overall opioid exposure and improving treatment outcomes in the first 72 hours after infusion suite discharge.
The findings reported herein are consistent with those of previously published studies that demonstrate the value of outpatient hematology/oncology infusion centers for reducing the time to first dose of opioid and frequency of pain reassessment4,8,10; decreasing ED visits, inpatient admissions, and hospital readmission rates4-10; and reducing costs to the health care system.6 Our study provides evidence that patient-specific treatment plans that recognize basic pharmacological principles can dramatically improve patient outcomes by reducing opioid use, shortening encounter duration, and reducing VOE relapse after discharge from the infusion center. An additional, unanticipated benefit of the short-interval infusion strategy is that it increases the capacity of the OEC to treat additional patients with VOE who would otherwise be evaluated in the ED, where both time to first infusion and clinical outcome are typically inferior. We are presently extending our studies to explore the benefits of embedding social work and psychological interventions into OEC treatment plans, as well as integrating infusion center pain plans with inpatient clinical care pathways, with the goal of improving multimodal care in the treatment of SCD in the ambulatory setting.
Acknowledgments: This work was supported by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Mentored Clinical Scientist Research Career Development Award K08 DK129716 (S.A.P.), Doris Duke Charitable Foundation Physician Scientist Fellowship Grant 2020062 (S.A.P.), ASH Scholar Award (S.A.P.), and NIH, National Institute of Nursing Research grant T32 NR009356 (A.M.F.).
Contribution: S.A.P., A.B.A., A.M.F., T.J.U., and F.A.S. designed the research study and wrote the manuscript; S.A.P., A.B.A., A.M.F., M.I., C.G., D.B., T.J.U., and F.A.S. performed the research and contributed essential data; S.A.P., A.B.A., A.M.F., J.E.R., T.J.U., and F.A.S. analyzed the data; and all authors critically revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Farzana A. Sayani, Hospital of the University of Pennsylvania, 3 Dulles Hematology, 3400 Spruce St, Philadelphia, PA 19104; e-mail: farzana.sayani@pennmedicine.upenn.edu.
References
Author notes
For original data, please contact farzana.sayani@pennmedicine.upenn.edu.
The full-text version of this article contains a data supplement.