Key Points
Overall survival of patients with cHL who progress after ASCT, approached 9.5 years in the novel agent era.
Patients who receive CPI as first treatment for post-ASCT progression had lower morality than patients who did not get novel agents.
Abstract
In the pre–novel agent era, the median postprogression overall survival (PPS) of patients with classic Hodgkin lymphoma (cHL) who progress after autologous stem cell transplant (ASCT) was 2 to 3 years. Recently, checkpoint inhibitors (CPI) and brentuximab vedotin (BV) have improved the depth and durability of response in this population. Here, we report the estimate of PPS in patients with relapsed cHL after ASCT in the era of CPI and BV. In this multicenter retrospective study of 15 participating institutions, adult patients with relapsed cHL after ASCT were included. Study objective was postprogression overall survival (PPS), defined as the time from posttransplant progression to death or last follow-up. Of 1158 patients who underwent ASCT, 367 had progressive disease. Median age was 34 years (range, 27-46) and 192 were male. Median PPS was 114.57 months (95% confidence interval [CI], 91-not achieved) or 9.5 years. In multivariate analysis, increasing age, progression within 6 months, and pre-ASCT positive positron emission tomography scan were associated with inferior PPS. When adjusted for these features, patients who received CPI, but not BV, as first treatment for post-ASCT progression had significantly higher PPS than the no CPI/no BV group (hazard ratio, 3.5; 95% CI, 1.6-7.8; P = .001). Receipt of allogeneic SCT (Allo-SCT) did not improve PPS. In the era of novel agents, progressive cHL after ASCT had long survival that compares favorably with previous reports. Patients who receive CPI as first treatment for progression had higher PPS. Receipt to Allo-SCT was not associated with PPS in this population.
Introduction
Classic Hodgkin lymphoma (cHL) is curable with frontline chemoimmunotherapy in 80% to 90% of cases, and 10% to 20% of cases relapse at 5 years.1 Salvage therapy and autologous stem cell transplant (ASCT) are standard of care in the treatment of relapse/refractory (R/R) cHL with 3 year freedom from treatment failure of 50% to 60%.2-4 Patients who are refractory to frontline therapy, who relapse within 1 year of frontline chemotherapy, who have pre-ASCT positive positron emission tomography (PET) scan, and who require multiple lines of salvage therapy are at particularly higher risk of disease relapse after ASCT.5-7 Patients who relapse after ASCT have traditionally had poor prognosis with a median survival of 2 to 3 years in the pre–novel agent era.8,9
Addition of novel agents, particularly brentuximab vedotin (BV) and checkpoint inhibitors (CPI) have shown encouraging response and survival in frontline and relapsed refractory disease.1,7,10-13 BV, combined with Adriamycin, dacarbazine, and vinblastine, has improved progression-free survival (PFS) and overall survival of cHL compared with longstanding standard of care chemotherapy regimen consisting of Adriamycin, vinblastine, bleomycin, and dacarbazine.1 In R/R cHL, salvage regimen incorporating BV and/or CPI has shown encouraging outcomes with 2-year PFS up to 97%.10,14 CPI-based regimens are associated with higher post-ASCT PFS than conventional savage chemotherapy in retrospective studies.7
In patients with cHL who relapse after ASCT, pembrolizumab monotherapy was compared with BV monotherapy in a randomized phase 3 trial and led to significantly higher median PFS of 11 months.15 Small retrospective studies of relapsed cHL after ASCT have reported improved posttransplant survival in the era of CPI and BV, but patients receiving novel agents were in minority.16-18 Large observational cohort study of the modern treatment era is needed to estimate posttransplant survival, prognostic factors, and the impact of sequencing of novel agents in cHL progressive after ASCT. Here, we report the outcomes of 367 patients with post-ASCT relapse of cHL using a multi-institutional observational cohort.
Methods
Patients and methods
Adult patients with R/R cHL who received systemic salvage therapies and underwent ASCT from 1 January 2011 to 31 December 2020 were enrolled in a multicenter cohort from 18 participating institutions. Fourteen centers were in the United States and 4 centers were in the Czech Republic. Patients with nodular lymphocyte predominant HL and patients who did not undergo ASCT were excluded. Of this parent cohort, all patients who had progressive disease after ASCT were included in this study. Demographic data (age and sex) at post-ASCT progression and clinical variables at pre-ASCT relapse (bulk, stage, primary refractory disease, early relapse, B symptoms, and extranodal disease) were recorded at each participating institution by electronic health review. Primary refractory disease was defined as inability to achieve complete remission after frontline therapy or progression within 3 months of completion of frontline therapy. Early relapse (ER) was defined as disease relapse within 12 months of completion of frontline therapy. Receipt of BV consolidation per ATHERA trial at the end of therapy was also recorded. Time to post-ASCT progression and the number and type of treatments received for post-ASCT progression were collected. From post-ASCT progression, participants were followed until death or last follow-up.
Data were collected by retrospective review of electronic health records at individual participating sites. After removing patient identifiers, data were transferred to Mayo Clinic that served as a leading site for data collection and analyses. Data transfer agreements were executed between Mayo Clinic and all the individual participating sites according to each institution’s requirements. Study was approved by the institutional review boards of each participating institution.
Objectives
Primary objective was postprogression overall survival (PPS), defined as the time from post-ASCT progression to death or last follow-up. Univariable and multivariate analyses were done to examine association of demographics and ATHERA risk factors (primary refractory disease, ER, extranodal disease, B symptoms, and pre-ASCT partial response, and ≥2 prior salvage therapies), as well as novel treatment strategies, with PPS.
Statistical analysis
Categorical variables were described using numbers and proportions. Continuous variables were described using median and range. Fisher exact test and χ2 test were used to detect the differences between variables. Kaplan-Meier estimates were used to describe time to event end points. Log rank test was used to compare Kaplan-Meier curves between groups. Univariable and multivariable associations with survival were tested using cox proportional hazard analysis and hazard ratio and 95% confidence interval (CI) were used to describe strength of associations. P value <0.05 was used to detect statistical significance. Nonrelapse mortality was defined as death without lymphoma relapse and was calculated from the date of post-ASCT progression in patients who underwent allogeneic SCT (Allo-SCT) after 1 line of postprogression therapy.
Results
Patient characteristics
Of the 1158 patients with R/R cHL who underwent ASCT, 367 patients had progressive disease after ASCT. Median age was 34 years (interquartile range, 27-46), and 192 patients were male. Median time to progression after ASCT was 6 months (range, 3-13 months), and 336 patients had at least 1 pre-ASCT modified ATHERA risk factor. A total of 65 patients (19%) relapsed during or after post-ASCT BV consolidation. Ninety patients (24%) received BV as pre-ASCT therapy; of whom, 14 (4%) also received CPI as pre-ASCT therapy. Median lines of therapy for post-ASCT progression were 2 (range, 1-9). A total of 147 patients (40%) received CPI at any point for post-ASCT progression, and 70 (20%) patients received CPI as a first line of treatment for post-ASCT progression. A total of 201 patients (55%) received BV at any point for post-ASCT progression, and 157 (43%) received BV as first post-ASCT progression treatment. One hundred seventeen patients (32%) were either treated with radiation alone (n = 39), chemotherapy with or without radiation (n = 59), other targeted agents such as everolimus or Panobinostat (n = 10), or received alloASCT (n = 9). Twenty-three patients did not have the name of agent available (supplemental Table 1).
Table 1 shows the characteristics of patients in the 3 major groups based on the first treatment (CPI, BV, or No CPI/BV) received at post-ASCT relapse. A higher number of patients in BV group received transplantation after 1 line of therapy, and a higher number of patients in no CPI/BV group received transplantation in partial response. A higher number of patients in the CPI-first group had time to progression (TTP) >6 months.
Outcomes
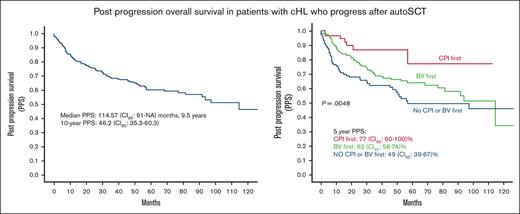
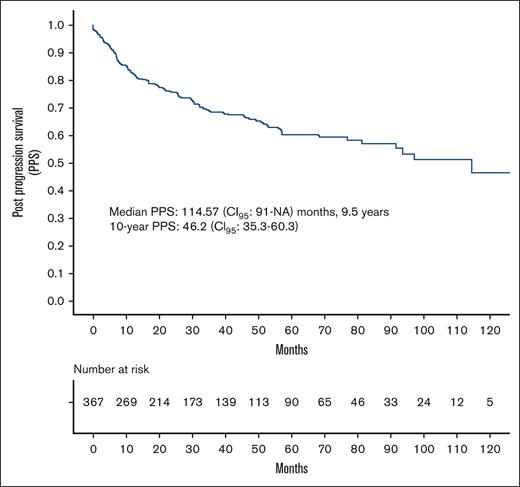
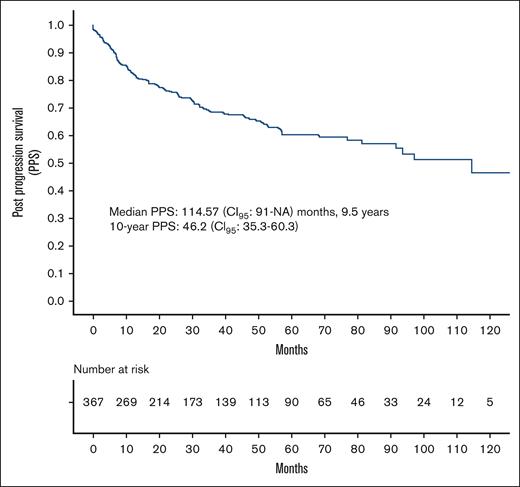
Median follow-up was 48.1 (range, 0.07-140) months. Median PPS was 114.57 (95% CI, 91-not achieved) months, that is, 9.5 years. Ten-year PPS was 46.2% (95% CI, 35.3-60.3) (Figure 1). There was no significant difference between PPS of the United States and the Czech Republic (supplemental Figure 1).
Factors associated with PPS
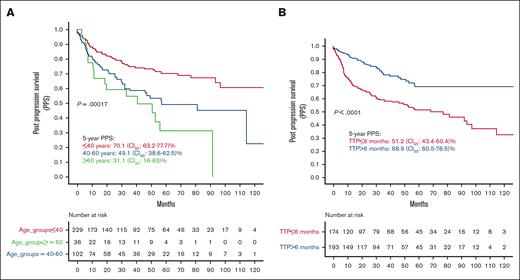
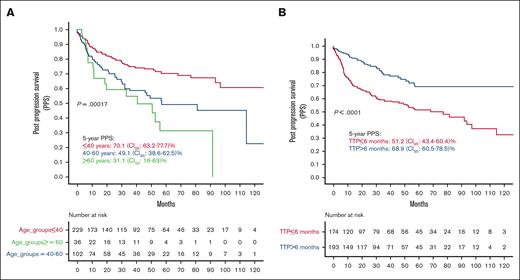
In the univariate analysis, increasing age was associated with worse PPS (Figure 2A). Patients aged <40 years had significantly higher PPS than those aged between 40 and 60 years and those aged >60 years (5-year PPS, 70.1; 95% CI, 63.2-77.7; 5-year PPS, 49.1; 95% CI, 38.6-62.5; and 5-year PPS, 31.1; 95% CI, 16-63, respectively; P = .00017). Patients with TTP within 6 months of ASCT had significantly inferior survival compared with patients who progressed after 6 months (5-year PPS, 51.2; 95% CI, 43.4-60.4 vs 5-year PPS, 68.9; 95% CI, 60.5-78.5, respectively; P < .0001; Figure 2B).
PPS by age group and time to posttransplant progression (TTP). (A) PPS by age groups. (B) PPS by TTP. NA, not achieved.
PPS by age group and time to posttransplant progression (TTP). (A) PPS by age groups. (B) PPS by TTP. NA, not achieved.
There was no difference between PPS of patients who received pre-ASCT BV or CPI (n = 90) and patients who were BV or CPI naïve at the time of post-ASCT progression (5-year PPS, 65%; 95% CI, 52-81 and 5-year PPS, 59%; 95% CI, 53-66; P = .9; supplemental Figure 2).
In addition to increasing age (hazard ratio [HR], 1.03; 95% CI, 1.01-1.04; P < .001) and TTP of ≤6 months (HR, 1.5; 95% CI, 1.03-2.18; P < .001), pre-ASCT high-risk features such as pre-ASCT positive PET scan (HR, 1.7; 95% CI, 1.1-2.6; P = .01), requirements of >1 salvage therapy (HR, 1.5; 95% CI, 1.07-2.3; P = .02) and B symptoms (HR, 1.5; 95% CI, 1.0-2.2; P = .02), and receipt of posttransplant BV maintenance (HR, 0.4; 95% CI, 0.2-0.9; P = .02) were associated with inferior PPS. Pre-ASCT extranodal disease, primary refractory disease, ER, and sex were not associated with PPS (Table 2).
In the multivariate analysis, increasing age, TTP of ≤6 months, and pre-ASCT positive PET scan were significantly associated with inferior PPS (Table 3).
Treatments for post-ASCT progression
We assessed how sequencing of BV and CPI treatment for posttransplant progression influences PPS. We divided the patients into 3 groups based on the first treatment received for post-ASCT progression: CPI group (n = 70), BV group (n = 157), and no CPI/BV group (n = 117). Twenty-three patients did not have details of treatments available and were excluded from this analysis. Of the 70 patients who received CPI as first treatment, 16 (23%) received further lines of treatment for recurrent disease. Of the 157 patients who received BV as first treatment, 76 (48%) received further treatment for recurrent disease. Of the 117 patients in no CPI/BV group, 77 (66%) received further treatment for recurrent disease. Patients in the CPI group had significantly lower likelihood of receiving further treatment for recurrent disease than those in the BV group (odds ratio [OR], 3.3; 95% CI, 1.3-6.8; P < .001) or those in the no CPI/BV group (OR, 5.1; 95% CI, 2.6-10.2; P < .001).
Of the 157 patients who received BV first, 54 (34%) received CPI at any point afterwards for disease recurrence. Of the 117 patients in the no CPI/BV group, 24 (20%) had CPI and 34 (29%) had BV as subsequent line of treatments. Of the 70 patients who received CPI first, 13 (19%) received BV as subsequent line of therapy.
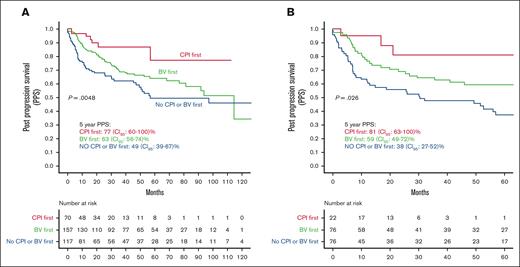
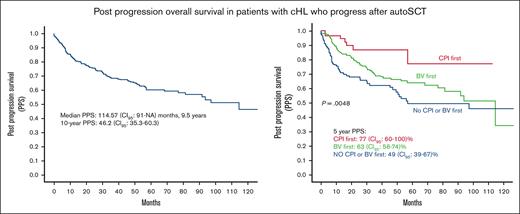
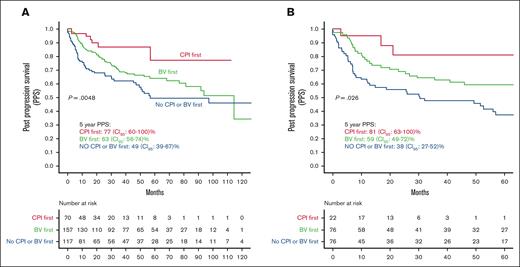
In the univariate analysis, patients who received CPI as the first treatment for post-ASCT progression had significantly higher PPS than those in the no CPI/BV group (HR, 3.4; 95% CI, 1.5-7.8; P = .002) and the BV group (HR, 2.4; 95% CI, 1.1-5.23; P = .03, Figure 3A). There was no difference in the PPS of patients who received BV first and those who did not receive CPI or BV as the first postprogression therapy (HR, 1.7; 95% CI, 0.96-2.0; P = .07). These results were consistent in the subgroup of patients who relapsed within 6 months of ASCT (Figure 3B).
PPS by type of first treatment for posttransplant progression. (A) In all available patients. (B) In patients who relapsed within 6 months.
PPS by type of first treatment for posttransplant progression. (A) In all available patients. (B) In patients who relapsed within 6 months.
When adjusted for age, TTP, and pre-ASCT PET response, patients in the CPI group consistently had superior PPS than those in the no CPI/BV group (HR, 2.6; 95% CI, 1.2-5.9; P = .01). There was no difference between the CPI and BV groups. (Table 4).
Radiation for post-ASCT progression
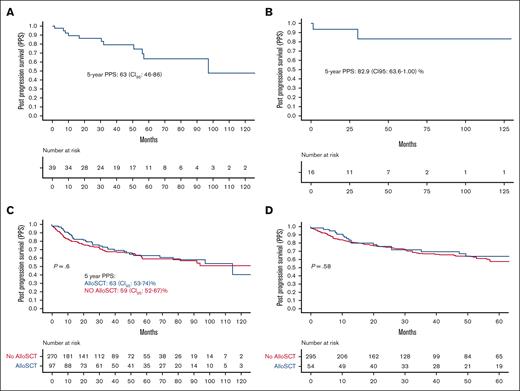
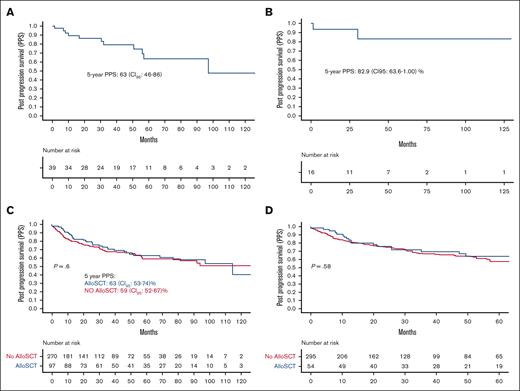
Thirty-nine patients received radiation only as first treatment at progression, 5-year PPS in this population was 63.3% (95% CI, 46.6-86.3) (Figure 4A). Sixteen patients (41%) stayed alive without requiring any subsequent therapy, and 5-year PPS in this population was 82.9% (95% CI, 63.6-1.00) (Figure 4B).
Outcomes of radiation and Allo-SCT. (A) PPS in patients who were treated with radiation only. (B) PPS in patients who were treated with radiation only and did not have disease recurrence. (C) PPS in patients who received Allo-SCT at any point. (D) PPS in patients who received Allo-SCT as a consolidation after first postprogression treatment.
Outcomes of radiation and Allo-SCT. (A) PPS in patients who were treated with radiation only. (B) PPS in patients who were treated with radiation only and did not have disease recurrence. (C) PPS in patients who received Allo-SCT at any point. (D) PPS in patients who received Allo-SCT as a consolidation after first postprogression treatment.
Receipt of Allo-SCT
Ninety-seven patients received Allo-SCT at any point after ASCT for disease progression, and 54 received Allo-SCT as consolidation to first treatment for post-ASCT progression. There was no difference in the characteristics of patients who did and those who did not receive Allo-SCT at any point (supplemental Table 2).
Of the 97 patients who were received Allo-SCT at any point, 19 (20%) required subsequent lines of therapy over a median follow-up of 25 months (range, 0.07-140). This was significantly lower than that of patients who did not receive Allo-SCT (102/270 [37%]; P = .001). There was no difference in the PPS of patients who received Allo-SCT at any point for post-ASCT progression vs patients who did not (Figure 4C). Twenty-five patients (39%) who received Allo-SCT died without any further relapse. In 54 patients who underwent Allo-SCT after 1 line of postprogression therapy, 49 did not require any further therapy for progression. Two-year probability of nonrelapse mortality in this population was 30% (95% CI, 15-40) (supplemental Figure 3).
To remove lead-time bias, we compared PPS of patients who received Allo-SCT as a consolidation therapy to first treatment for post-ASCT progression with those who did not. There was no significant difference between patients who received Allo-SCT as first treatment for progression after ASCT vs those who either received Allo-SCT for later lines of therapy or did not receive Allo-SCT at all (Figure 4D).
Discussion
In this multicenter cohort study, we observed that median PPS of patients with cHL relapsed after ASCT approaches 10 years in the modern treatment era. Increasing age, progression within 6 months of ASCT, and pre-ASCT positive PET scan were associated with inferior PPS. Patients who were given CPI first, but not BV first, had higher PPS than patients who did not receive BV or CPI first. Receipt of Allo-SCT was not associated with higher PPS.
Patients with cHL relapsed after ASCT have median PPS of 1 to 3 years in the pre–novel agent era.8,9 Retrospective studies have reported improved PPS in patients who were treated with CPI or BV for post-ASCT progression, although a smaller number of patients were treated with novel agents in these studies.16-18 A large study with majority of patients treated with novel agents would provide the estimate of prognosis of this population in the modern treatment era. The median survival of 9.5 years observed in this study compared favorably with reports from the pre–novel agent era. The observation validates previous findings and underscores efficacy of novel agents, both CPI and BV, in achieving durable remissions and improving outcomes in this population.
Increasing age and progression within 6 months of ASCT were associated with inferior survival in the pre–novel agent era.9 These factors continue to predict poor prognosis in the modern treatment era as observed in our study. This underscores the continued need for better treatment strategies in this patient population to improve outcomes, even in the modern treatment era.
We observed that pre-ASCT residual disease and B symptoms as well as requirement of multiple salvage therapies were associated with inferior survival even for patients who progressed after ASCT. This calls for better pre-ASCT treatment strategies in this high-risk subgroup that can reduce the likelihood of post-ASCT relapse. With increasing evidence that pre-ASCT CPI–based salvage therapies is associated with higher post-ASCT PFS, earlier use of CPI as a salvage regimen before ASCT could improve outcomes in this high-risk population.7,19
Patients who received CPI as first post-ASCT treatment had significantly higher PPS than patients who did not receive novel agents as first treatment and trend toward higher PPS than patients who received BV. This finding could be because of the longer duration of treatment in CPI arm than that in BV or chemotherapy arm. In addition, requirement of subsequent line of therapy was significantly lower in patients who received CPI first than those in other groups. Only a proportion of patients who did not receive CPI first were able to receive it as subsequent line of therapy. This underscores importance of earlier sequencing of CPI, even in the subgroup of patients who progressed within 6 months of ASCT, to improve outcomes.
Patients who received Allo-SCT had a significantly lower likelihood of requiring subsequent line of treatment but no difference in PPS than those who did not receive Allo-SCT. Furthermore, about one-fifth of patients who received Allo-SCT required subsequent line of therapy. Allo-SCT after PD1 blockade has been associated with substantial rates of graft-versus-host disease in retrospective studies.20 Taken together, this questions the utility of Allo-SCT in achieving cure or prolonging survival in patients with cHL relapsed after ASCT in the modern treatment era and might increase the risk of graft-versus-host disease. In the modern treatment era, Allo-SCT could probably be reserved for selective group of young patients with aggressive disease.
Peritransplant radiation has shown to improve outcomes in recurrent cHL but impact of palliative radiation in patients with post-ASCT relapse is not well described.21 We observed encouraging survival in a small group of patients who received local radiation only for post-ASCT progression, and about half of them had durable remissions without requiring further treatment. Thus, radiation might be efficacious in achieving durable remission in a selected group of individuals with low tumor burden at relapse.
Limitations of our study include retrospective nature and unavailability of disease features at post-ASCT progression. Lack of treatment duration and further relapses of postprogression therapies limit accurate analysis of duration of response. Despite these features, to our knowledge, our study provides the largest cohort till date, informing the outcomes of patients with cHL who relapsed after ASCT in the modern treatment era.
In conclusion, patients with cHL who progress after ASCT have a median survival of 9.5 years. Age >40 years, TTP <6 months, and less than complete metabolic response before transplant are associated with inferior PPS. Patients who received CPI but not BV had higher survival rate than patients who did not receive novel agents. This study reports outcomes of patients with cHL who progress after ASCT in a large cohort and serves as benchmark for future clinical trials.
Authorship
Contribution: S.H.D. and I.M. conceptualized and designed the study; S.H.D. and A.M.E. conducted statistical analyses; S.M.A., G.S.N., A.M.E., and I.M. reviewed the data and provided scientific input; S.H.D. wrote the manuscript; and all authors collected data and reviewed and approved the manuscript.
Conflict-of-interest disclosure: S.H.D. reports receiving honoraria from Seagen. M.A.S. reports honoraria from Notable Labs. V.B. reports membership on an entity’s board of directors or advisory committees and received research funding from FATE and Gamida Cell; research funding from Incyte; and membership on an entity’s board of directors or advisory committees in KaryoPharma. K.D. reports honoraria for SITC presentation and OncLive/Institutional Perspectives on Cancer presentation; research funding from Genmab, Janssen, F. Hoffman-La Roche Ltd, Juno/Bristol Myers Squibb, and Kite, a Gilead Company; and current employment at University of Pittsburgh/UPMC Hillman Cancer Center. S.A. reports research funding from Magenta Therapeutics. C.D. reports research funding/honoraria/consultancy fees from Celgene, IGM Biosciences, MEI, Janssen, Trillium, Seattle Genetics, Genentech, Inc/F. Hoffmann-La Roche Ltd, Incyte, Bristol Myers Squibb, AbbVie, Merck Sharp & Dohme, MorphoSys, and I-Mab; is a current equity holder in publicly-traded company Gilead; and is currently employed at Perlmutter Cancer Center at NYU Langone Health. G.S.N. reports research funding/consultancy fees from Celgene/Bristol Myers Squibb, Curis, Karyopharm Therapeutics, Bantham Pharmaceutical, Daiichi Sankyo, MorphoSys, Kite Pharma, Ryvu Therapeutics, Selvita, Genentech, Zai Laboratory, Incyte, Kymera Therapeutics, TG Therapeutics, Blueprint Medicines, Roche, and Nanostring. R.H.A. reports research funding/consultancy fees from Astellas/Agensys, Janssen Pharmaceutical, Kura, Merck, Millenium, Pharmacyclics, Portola Pharmaceuticals, Regeneron, and Seattle Genetics; membership on an entity’s board of directors or advisory committees in AstraZeneca, Bayer, Bristol Myers Squibb, Cell Medica, Gilead, Juno, Kite Pharma, Kyowa, Roche, Sanofi, and Takeda; and similar membership along with research funding from Forty Seven and Genetech Inc. S.M.A. reports research funding from Bristol Myers Squibb, Takeda, Seagen, ADC Therapeutics, Regeneron, Pfizer, Affimed, and AstraZeneca. A.M.E. reports honoraria from Seattle Genetics, Pharmacyclics, Research to Practice, Miltenyi Biotec, Epizyme, Novartis, MorphoSys, Cota Healthcare, Curio Science, Targeted Oncology, WebMD, AbbVie/Pharmacyclics, HMP, Takeda, Patient Power, PER, OncLive Clinical Congress Consultants, HUTCHMED, Incyte, MorphoSys, and Daiichi Sankyo/AstraZeneca; consulting or advisory role fees from Seattle Genetics, Novartis, Pharmacyclics, Miltenyi Biotec, Epizyme, MorphoSys, Cota Healthcare, AbbVie, Incyte, and MorphoSys; speaker’s bureau fees from Research to Practice and Curio Science; and travel, accommodations, and expenses fees from Seattle Genetics, Novartis, and Curio Science. The remaining authors declare no competing financial interests.
Correspondence: Sanjal H. Desai, University of Minnesota, 420 Delaware St SE MMC 480, Minneapolis, MN 55455; e-mail: desai171@umn.edu.
References
Author notes
Data related to this study are available upon reasonable request from the corresponding author, Sanjal H. Desai (desai171@umn.edu).
The full-text version of this article contains a data supplement.