Key Points
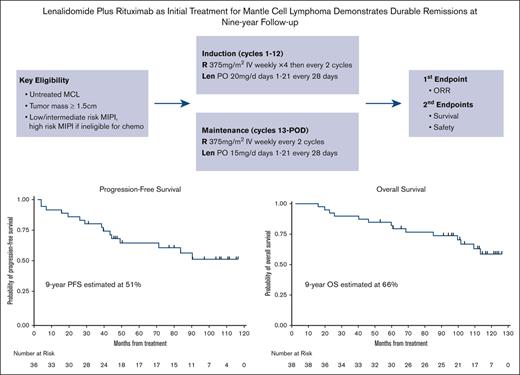
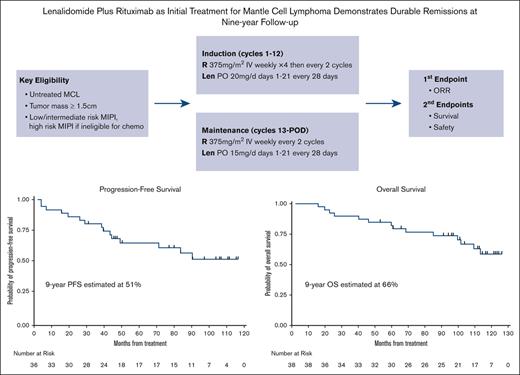
LR initial therapy for MCL provides durable efficacy with 9-year progression-free survival and overall survival of 51% and 66%, respectively.
Chronic maintenance treatment with LR doublet is feasible and safe with manageable side effects.
Abstract
Although chemoimmunotherapy is the current standard of care for initial treatment of mantle cell lymphoma (MCL), newer data suggest that there may be a role for a chemotherapy-free approach. We report the 9-year follow-up results of a multicenter, phase 2 study of lenalidomide plus rituximab (LR) as the initial treatment of MCL. The LR doublet is used as induction and maintenance until progression, with optional discontinuation after 3 years. We previously reported an overall response rate of 92% in evaluable patients, with 64% achieving a complete response. At a median follow-up of 103 months, 17 of 36 evaluable patients (47%) remain in remission. The 9-year progression-free survival and overall survival were 51% and 66%, respectively. During maintenance, hematologic adverse events included asymptomatic grade 3 or 4 cytopenia (42% neutropenia, 5% thrombocytopenia, and 3% anemia) and mostly grade 1 to 2 infections managed in the outpatient setting (50% upper respiratory infections, 21% urinary tract infections, 16% sinusitis, 16% cellulitis, and 13% pneumonia, with 5% requiring hospitalization). More patients developed grade 1 and 2 neuropathy during maintenance therapy (29%) than during induction therapy (8%). Twenty-one percent of patients developed secondary malignancies, including 5% with invasive malignancies, whereas the remainder were noninvasive skin cancers treated with local skin-directed therapy. Two patients permanently discontinued therapy because of concerns of immunosuppression during the COVID-19 pandemic. With long-term follow-up, LR continues to demonstrate prolonged, durable responses with manageable safety as initial induction therapy. This trial was registered at www.clinicaltrials.gov as #NCT01472562.
Introduction
Mantle cell lymphoma (MCL) is a distinct subtype of B-cell non-Hodgkin lymphoma characterized by t(11;14) translocations leading to cyclin D1 upregulation with a clinically heterogeneous disease course.1 Treatment of newly diagnosed MCL has traditionally involved chemoimmunotherapy, and for younger and physically fit patients, more intensive treatment regimens such as high-dose chemotherapy with hematopoietic stem cell transplantation are often used2-4 despite the lack of a likely cure. Selection of treatment takes account of disease burden, mutation profile, and patient characteristics, including age, comorbidities, and individual preferences. Many patients with MCL are older or have significant comorbidities, complicating their ability to tolerate intensive treatment, whereas others may present with high-risk mutations resistant to chemotherapy, highlighting the need for effective treatments with an efficacy and toxicity profile different from conventional chemotherapy, particularly in the frontline setting.5
Lenalidomide is a second-generation immunomodulatory agent that functions both by tumor microenvironment modifications and direct antilymphoma effects.6 It has been shown to stimulate T-cell and natural killer cell proliferation, induce lymphoma cell apoptosis via cyclin-dependent kinase inhibition and cyclin D1 downregulation, and inhibit tumor-associated lymphangiogenesis.7,8 In addition, the combination of lenalidomide plus rituximab (LR) has been shown to further augment targeted cell apoptosis through natural killer cell–mediated cytotoxicity9 and overcomes rituximab resistance in patients with lymphoma.10 Lenalidomide-based therapy has shown significant clinical activity against recurrent and treatment-refractory MCL as both a single-agent therapy (overall response rate [ORR], 28%-40%; complete response [CR], 5%-8%)11,12 and in combination with rituximab (ORR, 57%; CR, 36%).13
In 2011, we initiated a multicenter, phase 2 study to assess the efficacy and safety of combination LR as induction and maintenance treatment for patients with previously untreated MCL, the first published chemotherapy-free approach in this setting, to our knowledge. Previous analysis at a median follow-up of 30-months showed that the LR treatment was effective with an ORR of 92% and CR of 64%. It was moderately tolerated, and patients reported improvements in quality of life in response to therapy.14 During the 5-year follow-up, the LR regimen showed durable remissions, with a 5-year progression-free survival (PFS) of 64% and 5-year overall survival (OS) of 77%.15 The median PFS had not been reached at a median follow-up of 64 months, and measurable residual disease (MRD) assessment for 9 patients with CR showed that 8 had achieved MRD-negative status.
Since this study was published, there have been numerous investigations on novel agents and combinations as chemotherapy-free approaches in untreated MCL, with a few of the trials published. For example, a single-center phase 2 study at MD Anderson evaluated the combination of ibrutinib and rituximab, which showed an ORR of 96% with a CR rate of 71%.16 The GELTAMO group also recently published data combining ibrutinib with rituximab in indolent MCL and demonstrated an ORR of 84% and an 80% CR rate, with 87% achieving negative minimal residual disease testing in the blood.17 One arm of the OAsIs study assessed ibrutinib with venetoclax, a B-cell lymphoma 2 protein inhibitor, and obinutuzumab, an anti-CD20 antibody, in patients with untreated MCL, showing an ORR of 100% and CR of 47%. Grade 3 or 4 toxicities included lymphocytosis, neutropenia, and hepatobiliary toxicity.18 Still, these studies have limited long-term follow-up. Taken together, these data suggest an alternative to standard chemotherapy approaches and highlight the potential of chemotherapy-free treatment for patients with untreated MCL.
Here, we report the 9-year follow-up on the efficacy and safety of the LR regimen as well as the impact of the COVID-19 pandemic on the patients on the trial.
Methods
Patient eligibility
Details on patient eligibility were reported in previous publications.14,15 Briefly, the eligibility criteria included measurable, histologically confirmed, untreated MCL. A low- to intermediate-risk MCL International Prognostic Index (MIPI) score or high-risk MIPI score with contraindications to chemotherapy was also required. Patients were required to have an Eastern Cooperative Oncology Group performance status score ≤ 2 and creatinine clearance ≥ 30 mL/min. Patients were excluded if they had central nervous system lymphoma, known HIV infection, active hepatitis B or C infection, or invasive malignant tumors within 5 years before the start of treatment.
Study design
Details on study design were published previously.14,15 As a brief summary, this multicenter, open-label, single-arm study consisted of induction and maintenance phases. Lenalidomide was administered at 20 mg daily for the first 21 days of a 28-day cycle for 12 cycles during the induction phase, with dose escalation to 25 mg daily after the first cycle as tolerated. For maintenance, lenalidomide dosage was reduced to 15 mg daily. For patients with creatinine clearance between 30 and 60 mL/min, the dose of lenalidomide was adjusted to 10 mg daily for induction and 5 mg daily for maintenance. Rituximab was administered at 375 mg/m2 weekly during the 4 weeks of cycle 1 and then administered once every other cycle, including during maintenance. Treatment was continuous until progression of disease, development of unacceptable adverse events, or voluntary withdrawal from study. Patients may opt to stop treatment after 3 years if they were in clinical remission based on computed tomography (CT). The study was conducted in accordance with the Declaration of Helsinki and good clinical practice guidelines. Institutional review boards approved the study protocol at the respective study sites. All participants provided written informed consent. An independent data and safety monitoring board at Weill Cornell conducted biannual safety reviews.
Efficacy and safety assessment
Response to treatment was determined using the Cheson criteria.19 CTs were performed at baseline, every 3 months for 2 years of treatment, and every 6 months until disease progression. To confirm CR, a bone marrow biopsy and positron emission tomography–CT were performed. Adverse events were monitored throughout the study, and the toxicities were graded per National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
Statistical analysis
Details in regard to the determination of sample size have been described previously.14 In summary, the primary end point for the study was ORR (CR plus partial response). The sample size was determined based on a Simon 2-stage minimax design, and the final accrual was 38 patients (36 patients available for response assessment). Secondary end points of interest were PFS and OS, which were assessed by Kaplan-Meier survival analysis. P values were 2 sided, and statistical significance was attributed to values <.05. All analyses were performed with the use of SAS software (SAS Institute), and Stata software (StataCorp).
Results
Patient characteristics and disposition
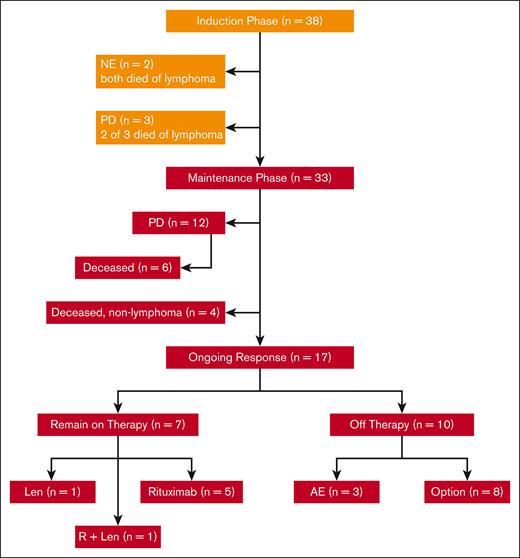
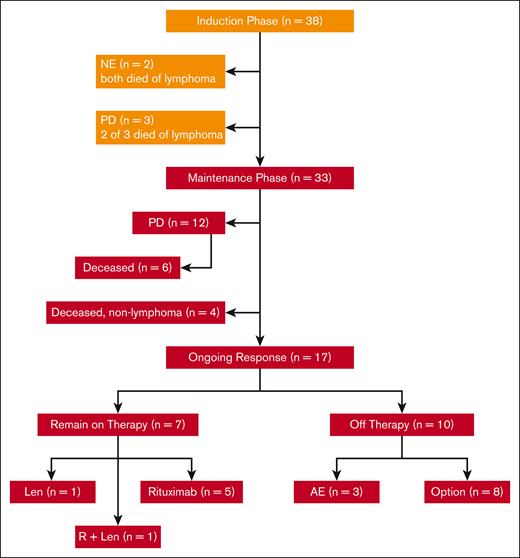
In total, 38 patients with untreated MCL requiring therapy were enrolled at 4 centers between July 2011 and April 2014, as reported in prior publications.14,15 Their features were typical for patients with newly diagnosed MCL, including a median age of 65 years (range, 42-86 years), advanced stage, and evenly distributed MIPI scores between low, intermediate, and high risk. Of the 87% of patients with evaluable Ki67 marker, 21% had Ki67 of >30% and none had pleomorphic or blastoid histology. Two patients withdrew from the study and were not evaluable before response assessment because of tumor flare; both eventually died from lymphoma after other treatment regimens. Of 36 patients who were evaluable for response, 33 completed the induction phase of therapy and started maintenance therapy with LR (Figure 1). A total of 15 patients had progression of disease: 3 during induction therapy with primary refractory disease, and 12 during maintenance after initial response. Of the patients who progressed during maintenance, 6 had initial CRs with PFS of 18, 38, 39, 49, 72, and 85 months, respectively, and 6 had initial partial responses with progression at 14, 25, 28, 43, 44, and 92 months, respectively.
CONSORT diagram of patient treatment and disposition. The induction treatment consisted of lenalidomide (Len) administered at 20 mg daily on days 1 to 21 of a 28-day cycle for 12 cycles and rituximab (R) weekly for 4 weeks during cycle 1 and then every other cycle. Of the 38 patients enrolled, 33 completed induction and entered maintenance, whereas Len was reduced to 15 mg, and R was continued every other cycle. Treatment was continuous until disease progression, unacceptable toxicity, or study withdrawal, with an option to stop therapy after 3 years. AE, adverse event; NE, not evaluable; PD, progressive disease.
CONSORT diagram of patient treatment and disposition. The induction treatment consisted of lenalidomide (Len) administered at 20 mg daily on days 1 to 21 of a 28-day cycle for 12 cycles and rituximab (R) weekly for 4 weeks during cycle 1 and then every other cycle. Of the 38 patients enrolled, 33 completed induction and entered maintenance, whereas Len was reduced to 15 mg, and R was continued every other cycle. Treatment was continuous until disease progression, unacceptable toxicity, or study withdrawal, with an option to stop therapy after 3 years. AE, adverse event; NE, not evaluable; PD, progressive disease.
Thus far, 12 evaluable patients have died, 7 from lymphoma progression (including 1 who relapsed 49 months after completion of 3-year study treatment), and 5 from nonlymphoma causes including heart failure, West Nile virus infection, pancreatic cancer, and COVID-19 pneumonia. As of June 2022, with a median follow-up of 103 months (8.6 years), 17 evaluable patients remain in remission. Of these patients in remission, 7 patients remain on study treatment, including 5 on rituximab maintenance, 1 on lenalidomide alone, and 1 on LR (Figure 2), whereas 10 patients discontinued study treatment (Figure 2; Table 1).
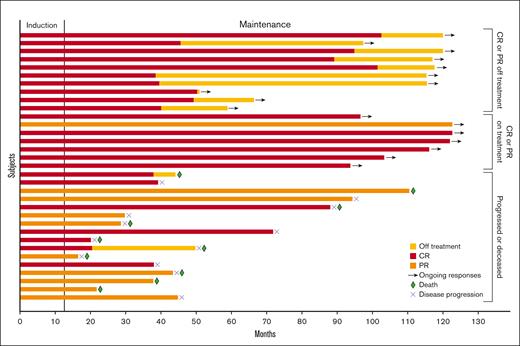
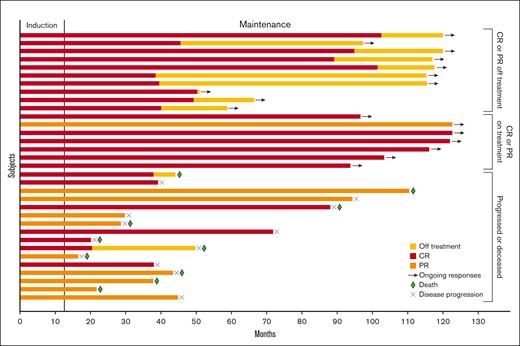
Swimmer plot of response duration. In total, 33 patients had partial response (PR) or CR on study treatment. Bar length indicates duration of response. The segment to the left of the horizontal line indicates the induction phase, which consisted of 12 cycles of treatment. Red, orange, and yellow bars indicate CR, PR, and time off treatment, respectively. Arrows, green diamonds, and blue Xs indicate ongoing response, death, and disease progression, respectively.
Swimmer plot of response duration. In total, 33 patients had partial response (PR) or CR on study treatment. Bar length indicates duration of response. The segment to the left of the horizontal line indicates the induction phase, which consisted of 12 cycles of treatment. Red, orange, and yellow bars indicate CR, PR, and time off treatment, respectively. Arrows, green diamonds, and blue Xs indicate ongoing response, death, and disease progression, respectively.
Efficacy
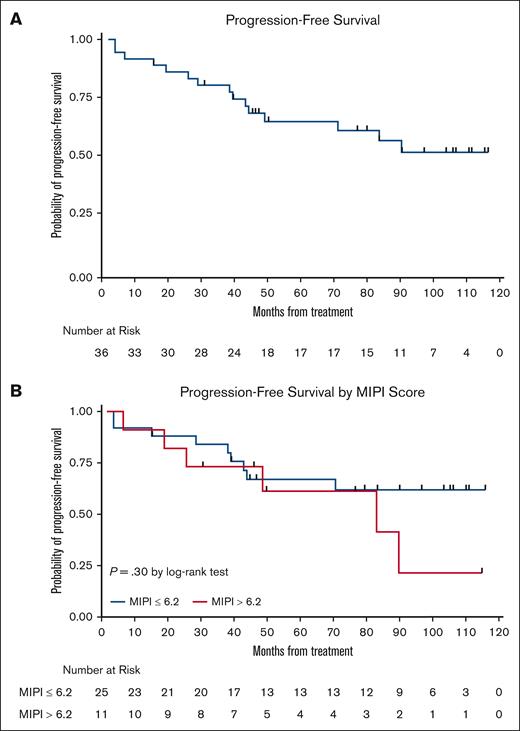
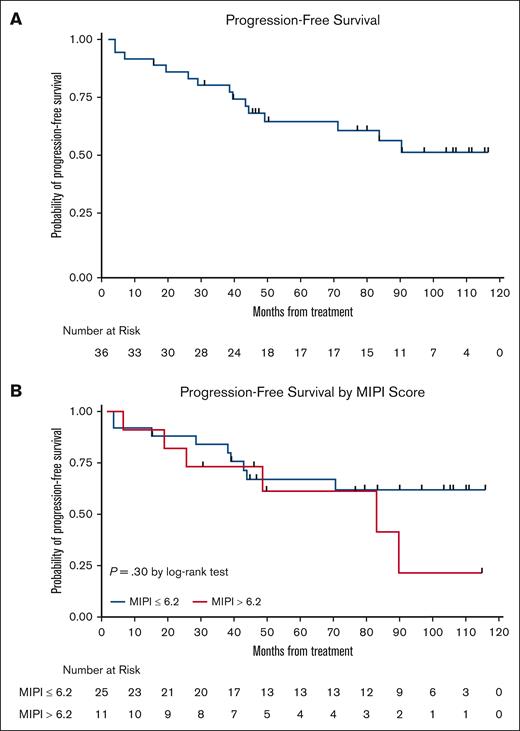
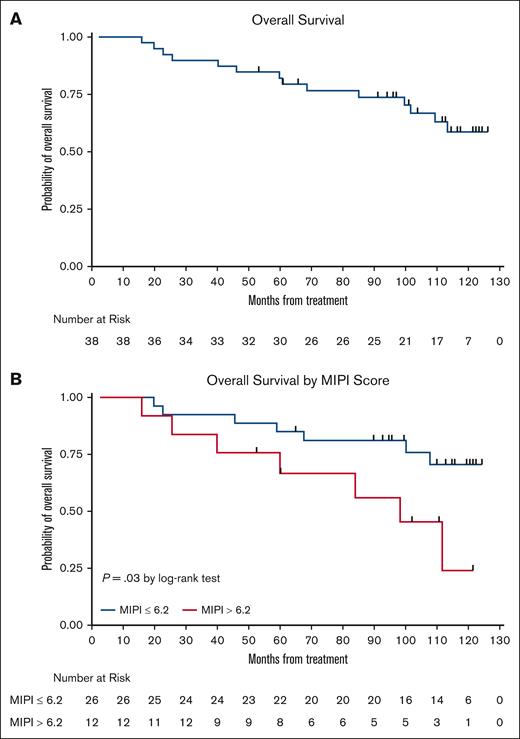
The LR combination produced excellent response rates, as reported previously.15 At final analysis, the median PFS was 9 years (Figure 3), with 17 responses ongoing, including 17 beyond 6 years, 15 beyond 7 years, 11 beyond 8 years, and 6 patients beyond 9 years. The 7-year and 9-year PFS rates were estimated at 61% (95% confidence interval [CI], 41.8-75.2) and 51% (95% CI, 31.5-68.0), respectively (Figures 3 and 4). The 7-year and 9-year OS rates were estimated at 76% (95% CI, 58.7-86.7) and 66% (95% CI, 47.3-79.2), respectively. During maintenance, 12 patients in complete remission were able to discontinue study treatment and durable remissions were maintained in 10 patients (Table 1). MIPI scores were not associated with either response or PFS (Figure 3); however, high-risk MIPI scores were associated with a less favorable OS (P = .03; Figure 4). As with prior publication, Ki67 > 30% did not have an impact on either PFS or OS.
Kaplan-Meier PFS curves. (A) PFS curve; and (B) PFS stratification based on MIPI.
Kaplan-Meier PFS curves. (A) PFS curve; and (B) PFS stratification based on MIPI.
Kaplan-Meier OS curves. (A) OS curve; and (B) OS stratification based on MIPI.
Toxicities
As reported in the initial report and subsequent 5-year follow-up, patients receiving long-term therapy were monitored closely during maintenance (Table 2). Grade 3 or greater hematologic toxicities, including neutropenia (42%), anemia (3%), and thrombocytopenia (5%) were both less frequent and less intense than during induction therapy; 2 developed febrile neutropenia (5%) that resolved with IV antibiotics and granulocyte colony-stimulating factor. Infections were mostly grade 1 to 2 and represented upper respiratory infections (50%), urinary tract infections (21%), sinusitis (16%), and cellulitis (16%). Pneumonia occurred in 13%, including 2 requiring hospitalization (5%).
There were very few grade 3 or greater nonhematologic toxicities during maintenance therapy, with 1 patient developing grade 3 aspartate transferase and alanine transaminase elevations and 1 upper respiratory infection requiring hospitalization. More patients developed grade 1 and 2 neuropathy during maintenance therapy (29%) than during induction therapy (8%).
Secondary primary malignancies
A total of 8 patients (21%) reported secondary primary malignancies. Two patients (5%) developed invasive systemic malignancies: 1 developed a Merkel cell carcinoma after 20 months of therapy (and developed melanoma in-situ as well), and the other patient developed pancreatic cancer after 12 months on therapy. Both patients passed away from their secondary malignancies. The remainder cases were noninvasive skin cancers treated with local skin-directed therapy without the need for study interruption.
Treatment modifications
As previously reported, during induction, the median dose of lenalidomide was 20 mg for patients with normal renal function, including 42% requiring dose reduction from 20 mg, and 35% tolerating escalation from 20 to 25 mg. Three patients discontinued lenalidomide at the completion of induction therapy before starting maintenance because of adverse events. In total, 70% of patients required dose reduction from 15 mg during the maintenance phase, with a median maintenance dose of 10 mg. Per the current follow-up, 7 patients remain on therapy including 5 on rituximab alone, 1 on lenalidomide alone, and 1 on LR.
Effects of the COVID-19 pandemic
In total, 4 patients developed COVID-19 infection while on study (Table 2). Of these patients, 1 patient was hospitalized and succumbed to COVID-19 infection; 3 other patients developed COVID-19 but did not require hospitalization, 1 of which had COVID-19 twice, in February and December 2021. All 4 initial infections were before patients received severe acute respiratory syndrome coronavirus 2 vaccination. All patients’ therapy was held during active infection; 3 patients held maintenance rituximab as a precaution in March 2020, and 1 resumed maintenance rituximab in May 2020 whereas the other 2 permanently discontinued it.
Discussion
Our study set out to investigate the efficacy of first-line MCL treatment with LR in order to offer an alternative to frontline chemoimmunotherapy. In the extended 9-year follow-up of this study, we demonstrate that LR in the first-line treatment of MCL produced high response rates (ORR, 91.7%; CR, 46.2%) and durable remissions relative to those of conventional chemoimmunotherapy (9-year PFS, 51.3%; 9-year OS, 65.8%). We also found that both induction and maintenance therapy with LR is feasible and effective. Importantly, among 17 patients who are in remission, 10 have maintained durable responses after therapy discontinuation.
Patients involved in this study had a median age of 65 years, and 68% of patients had intermediate/high MIPI scores, demonstrating the applicability of our findings to patients typically presenting for treatment of MCL. Adverse events observed in this study included primarily asymptomatic cytopenia, infections of grades 1 to 2, and inflammatory symptoms. These adverse events are comparable with those that have been observed in prior studies in which LR have been used for relapsed/refractory MCL11-13 and were tolerable in the vast majority of patients with long-term administration (treatment stopped because of adverse events in 6 of 38 patients [16%]). With respect to secondary primary malignancies, which is a concern with lenalidomide-based therapy, 21% of patients developed secondary malignancies, including 5% with invasive malignancies, whereas the remainder were noninvasive skin cancers treated with local skin-directed therapy. We observed no instance of hematologic malignancies, including acute myeloid leukemia or myelodysplastic syndrome.
Although head-to-head data regarding various frontline regimens in MCL are lacking, and comparison with historical phase 2 or 3 chemoimmunotherapy–based data has limitations with regard to disease features and patient selection, the PFS and OS of patients who have received LR as frontline study treatment seem to be comparable with and, in some cases, exceed the historical outcomes of those who received outpatient-based frontline regimens in the same era. These include the phase 3 StiL NHL1 trial, which demonstrated a median PFS of 35 months for patients treated with endamustine + rituximab (BR) frontline regimen20; the phase 3 BRIGHT study, which demonstrated a PFS rate of 70% at 5 years with BR21; the LYM-3002 trial, which demonstrated a median PFS of 24.7 months and median OS of 91 months in the bortezomib + rituximab–cyclophosphamide, doxorubicin, and prednisone arm22; and the phase 3 MCL Elderly study, which demonstrated median PFS and OS of 5.4 and 9.8 years, respectively, with rituximab–cyclophosphamide, doxorubicin, vincristine, and prednisone induction and rituximab maintenance.23
The use of LR maintenance therapy for patients who are ineligible to receive transplantation is promising and has been investigated in other studies. The MCL R2 Elderly trial randomized patients to undergo frontline treatment with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone followed by rituximab or LR maintenance. At a median follow-up of 2.1 years, the 2-year PFS rates were 76.6% and 60.8% among patients receiving LR maintenance and rituximab maintenance, respectively, favoring LR maintenance.24 The E1411 trial randomized patients to 4 arms to undergo frontline treatment of MCL with BR or BVR induction followed by rituximab or LR maintenance. The estimated PFS at 2 years was not significantly different between LR or rituximab maintenance arms (85.8% LR vs 77.7% rituximab), suggesting that response quality from induction regimens may affect the outcomes of maintenance.25
At the time that the LR study was designed, there were no data available on the optimal duration of maintenance therapy with the frontline chemotherapy-free approach. Our data have indicated that in 17 long-term responders, 10 subjects were able to discontinue study treatment and enjoy ongoing remissions. Future studies are needed to examine the feasibility of limited-duration maintenance therapy tailored to disease risks and response qualities to minimize treatment-related toxicities while maintaining efficacy.
Bruton tyrosine kinase (BTK) inhibitor–based combinations are now driving the clinical development of chemotherapy-free options, and the results of ongoing phase 1, 2, and 3 trials will help to establish the next era. The ibrutinib-rituximab doublet delivered first-line showed good efficacy, with an ORR of 82% to 98%, CR of 60% to 75%, and MRD-negative rate of 81% to 87% with CR.16,17 Newer combinations under investigation include next-generation BTK inhibitor combinations such as acalabrutinib-rituximab (ALTAMIRA; NCT05214183), acalabrutinib-LR (NCT03863184), acalabrutinib-venetoclax-rituximab (NCT02717624), and zanubrutinib-obinutuzumab-venetoclax (NCT03824483), among others. Some of these studies have begun to address response-adaptive treatment strategy as well as biomarker-driven risk stratification. For example, the GELTAMO IMCL-2015 study experimented time-limited treatment of 2 years for patients who achieved MRD-undetectable CR.17 Similarly, the ongoing acalabrutinib-LR study allowed study treatment discontinuation for patients with MRD-undetectable molecular remission during maintenance to minimize treatment-related side effects.26 The phase 2 BOVEN trial, tailored to patients with TP53-mutated high-risk MCL, has promising initial data, with an ORR of 86% and CR rate of 64% at a median follow-up of 4 months.27 Importantly, the phase 3 ENRICH and MANGROVE trials (ISRCTN11038174 and NCT04002297, respectively) are ongoing, directly comparing the efficacy of BTK inhibitor (either ibrutinib or zanubrutinib, respectively) plus rituximab with that of chemoimmunotherapy, with outcomes expected of potential practice-changing impact.
Novel agents deliver immense potential in terms of efficacy, convenience, and a different side-effect profile, and further studies are necessary to better understand the role of their combination in first-line treatment. Delivering increasingly personalized therapy based on patients’ mutational status such as TP53 and on MRD response on therapy is also needed to advance care by maximizing efficacious treatments and minimizing treatment-related toxicities.
The LR study is the first chemotherapy-free frontline treatment for MCL, and our report provides the long-term data using a chemotherapy-free approach in MCL. The efficacy of first-line LR, as evidenced by high response rates and durable remissions as well as long-term safety with the convenience of an outpatient treatment regimen support the broad-based applicability of this regimen as a novel approach to previously untreated MCL.
Acknowledgments
The authors thank Lubing Wu, Brittany Hobbie, and Tejasvi Kaur Sahni for assistance in patient data collection.
This study was supported, in part, by Bristol Myers Squibb (Celgene) and a Clinical Translational Science Center grant (1-UL1-TR002384-01) (P.C.).
Authorship
Contribution: S.Y. coordinated data interpretation and wrote the manuscript; J.R. designed the study, participated in patient care and data analysis, and wrote the manuscript; G.Z.C. and C.G. participated in data collection and analysis and in writing of the manuscript; J.P.L., P.M., B.S., S.J.S, S.M.S., R.R.F., and J.S. contributed to the logistic support of the study, patient care, and critically reviewed the manuscript; and P.C. performed biostatistical analysis of patient and biomarker data.
Conflict-of-interest disclosure: B.S. has provided consulting advice and education to Amgen, Pfizer, Novartis, Bristol Myers Squibb (BMS), Kite/Gilead, Precision Biosciences, Jazz, BeiGene, Adaptive, Century Therapeutics, Autolus, Deciphera, Lilly, Takeda, and AstraZeneca. J.S. has provided consulting advice to Seagen, BMS, AstraZeneca, Pharmacyclics, Adaptive Biotechnologies, and Atara and received research funding from Seagen, Celgene, Pharmacyclics, Merck, BMS, Incyte, AstraZeneca, and Adaptive Biotechnologies. R.R.F. has provided consulting advice to Genentech. J.P.L. has provided consulting advice to AbbVie, Astellas, AstraZeneca, Bayer, BeiGene, BMS, Calithera, Constellation, Eisai, Lilly, Epizyme, Genmab, Grail, Incyte, Jansssen, Karyopharm, Lilly, Merck, Mustang Bio, Pfizer, Roche/Genentech, Second Genome, and Sutro. P.M. has provided consulting advice to AbbVie, AstraZeneca, BeiGene, Daiichi, Sankyo, Ipsen, Roche-Genentech, Janssen, Merck, and Pepromene. J.R. has received research support from BMS/Celgene, AstraZeneca, Genentech, and Daiichi Sankyo as well as honorarium for service as a consultant for Secura Bio, Daiichi Sankyo, AstraZeneca, Janssen, and Kite Pharma. The remaining authors declare no competing financial interests.
Correspondence: Jia Ruan, Meyer Cancer Center, Weill Cornell Medicine, 1305 York Ave, 7th Floor, New York, NY 10021; e-mail: jruan@med.cornell.edu.
References
Author notes
Data are available on request from the corresponding author, Jia Ruan (jruan@med.cornell.edu).