Abstract
Postpartum hemorrhage (PPH) is a leading cause of maternal morbi-mortality. Although obstetric risk factors are well described, the impact of predelivery hematologic and hemostatic biomarkers remains incompletely understood. In this systematic review, we aimed to summarize the available literature on the association between predelivery hemostatic biomarkers and PPH/severe PPH. Searching MEDLINE, EMBASE, and CENTRAL databases from inception to October 2022, we included observational studies on unselected pregnant women without bleeding disorder reporting on PPH and on predelivery hemostatic biomarkers. Two review authors independently performed title, abstract and full-text screening, upon which quantitative syntheses of studies reporting on the same hemostatic biomarker were conducted, calculating the mean difference (MD) between women with PPH/severe PPH and controls. A search on 18 October 2022 yielded 81 articles fitting our inclusion criteria. The heterogeneity between studies was considerable. With regard to PPH, the estimated average MD in the investigated biomarkers (platelets, fibrinogen, hemoglobin, Ddimer, activated partial thromboplastin time, and prothrombin time) were not statistically significant. Women who developed severe PPH had lower predelivery platelets than controls (MD = −26.0 109/L; 95% confidence interval, −35.8 to −16.1), whereas differences in predelivery fibrinogen concentration (MD = −0.31 g/L; 95% confidence interval, −0.75 to 0.13) and levels of factor XIII or hemoglobin were not statistically significant in women with and without severe PPH. Predelivery platelet counts were, on average, lower in women with severe PPH compared with controls, suggesting the potential usefulness of this biomarker for predicting severe PPH. This trial was registered at the International Prospective Register of Systematic Reviews as CRD42022368075.
Introduction
Postpartum hemorrhage (PPH) affects between 5% and 10% of woman who are delivering and is still a leading cause of maternal morbidity and mortality during childbirth worldwide.1 This condition occurs very suddenly and is associated with predisposing factors, some of which are related to baseline maternal characteristics (eg, obesity and age >35 years) and others to pregnancy characteristics (assisted reproductive technology, multiple pregnancies, abnormal placental insertion, or preeclampsia) or delivery characteristics (cesarean delivery [C-section], episiotomy, duration of labor, oxytocin use during labor, and macrosomia).2 However, in a significant number of women experiencing PPH, no risk factor can be identified before delivery, and ∼20% of PPH cases remain unexplained, which complicates the prediction and, thus, the prevention of PPH.
Coagulation disorders can directly cause PPH. In addition, excessive bleeding leads to consumption of coagulation factors, which may be exacerbated via dilutional coagulopathy after volume resuscitation, and may lead to the progression of PPH to severe PPH.3
Several studies have focused on clinical and biological parameter analyses incorporated in predictive scores to predict PPH. However, none of these predictive scores is implemented for use in daily practice worldwide, especially because of their limited discriminatory abilities and the lack of robust external validation.4,5
Because women at risk of PPH should ideally be identified before delivery, we focused our literature analysis on biological parameters that had been obtained before delivery and conducted a systematic review aiming at synthetizing available data on hemostatic biomarkers measured before delivery as potential predictors of PPH. The results of this work could guide preventive strategies and future research.
Methods
We conducted a systematic review of the literature on predelivery hemostatic biomarkers associated with PPH and/or with severe PPH.
The study protocol was prepared before the initiation of the literature research, adhering to the Preferred Reporting Items for Systematic review and Meta-Analysis (PRISMA) protocols 2015 statement and was registered at PROSPERO (the International Prospective Register of Systematic Reviews) before study initiation, on 18 October 2022 (protocol ID, CRD42022368075).
The study was conducted and presented following the PRISMA protocols and the guidelines for reporting meta-analysis of observational studies in epidemiology.
Search strategy
We performed a systematic search of the literature using EMBASE, MEDLINE, and the Cochrane Library (CENTRAL) from their inception to October 2022.
Search terms for the literature review were predefined and included (supplemental Material): postpartum h(a)emorrhage, postpartum bleeding, postpartum bleed, postnatal h(a)emorrhage; platelets, platelet count, thrombocytopenia, hemoglobin, an(a)emia, fibrinogen, factor II, prothrombin, factor V, factor VII, factor VIII, factor IX, factor X, factor XI, factor XII, factor XIII, von Willebrand factor, antithrombin, protein C, protein S, tissue factor pathway inhibitor, plasminogen activator inhibitors (PAIs), thrombin activatable fibrinolysis inhibitor, carboxypeptidase B2, prothrombin time (PT), activated partial thromboplastin time (aPTT), fibrin fragment D, fibrin fibrinogen degradation products, D-dimer, thrombin, fibrinolysis, fibrinolysin, plasminogen, plasmin, thromboelastography, TEG, thromboelastometry, ROTEM, viscoelastic assay, fibrin clot lysis time, and clot formation.
Two researchers (C.d.M. and D.M.) independently identified studies eligible for inclusion, based on screening titles and abstracts available in English. Duplicate search results were excluded before eligibility screening. Full-text articles, letters, brief reports, editorials, and correspondences were eligible for inclusion. Search for additional studies not identified by the search criteria was conducted manually. Inclusion of a study was based on consensus of its suitability by the aforementioned 2 researchers. The reasons for exclusion of full-text articles were documented. The process of study selection is displayed using a PRISMA flow diagram.
Risk of bias evaluation
Quality assessment of the study design and methodology of identified studies was performed independently by 2 researchers (C.d.M. and D.M.). Risk of bias was assessed using the Newcastle-Ottawa quality assessment scale, a validated tool for the assessment of the risk of bias in nonrandomized studies.
Inclusion criteria
The following criteria were determined for inclusion of a study: unselected pregnant women, reporting of PPH as an outcome (either PPH in general or only severe PPH), and observational study (prospective and retrospective cohort studies, cross-sectional studies, and case-control studies).
Exclusion criteria
Studies that exclusively reported on a selected population such as women with a known bleeding disorder (eg, Von Willebrand disease, hemophilia carrier, coagulation factor deficiency, congenital thrombopathy, chronic thrombocytopenia because of immunological disorders [eg, immune thrombocytopenic purpura] or because of other chronic diseases present before pregnancy) and of pregnant women diagnosed with hemolysis, elevated liver enzymes, and low platelet (HELLP) syndrome, or those with pre-eclampsia were excluded.
Besides these, case-series studies and case-reports were excluded.
Data extraction
Studies compatible with the predefined inclusion criteria and that did not fulfill any exclusion criteria upon consensus of the 2 researchers (C.d.M. and D.M.) underwent data extraction. Data extraction of predefined baseline and outcome variables was then performed as described hereafter. All data were independently extracted from eligible studies by the 2 researchers (C.d.M. and D.M.), with inconsistencies resolved by discussion with a third researcher (S.N.).
The following data were collected from full-text publications of included studies: study identifiers (first author, publication year, title, and country); study-specific methodological data (sample size, study design, and monocenter vs multicenter); study population–specific data (demographics [age and body mass index], preexisting comorbidities, obstetric history [nulliparous, multiparous, PPH history, and previous C-section], smoking status during pregnancy, type of pregnancy [singleton vs multiple pregnancies and assisted conception vs natural], abnormal placental insertion [placenta previa, accreta, or percreta], pregnancy complications [gestational hypertension, preeclampsia, HELLP syndrome, and intrauterine growth restriction, stillbirth, antepartum hemorrhage, placental abruption, chorioamniotitis, venous thromboembolism, premature rupture of membranes, or gestational diabetes], mode of delivery [vaginal delivery vs elective C-section or emergency C-section, labor induction, duration of labor, oxytocin use during labor, and type of analgesia], the term at delivery, retained placenta, amniotic fluid embolism, and newborn birth weight/macrosomia [≥4000 g]); biologic-specific data (the time at blood collection [in weeks of gestation trimester of gestation, or at the entry in the delivery room]), unit of measurement, type of measurement (concentration vs activity or rate [%]), mean with standard deviation [SD], median with interquartile range and range for PPH group and for control group, cutoff values, odds ratios or hazard ratios for PPH with 95% confidence interval [CI]); and outcome-specific data (prevalence of PPH in the studied population, PPH severity [based on respective definition; in particular, in case of severe PPH: the need for blood transfusion, need for uterine artery embolization, need for hysterectomy, hemorrhagic shock, need for intensive care, or related death], and the main cause of PPH [uterine atony, placental cause, traumatic cause, or coagulation disorder]).
Hemostatic biomarkers of interest
Predefined biomarkers of interest were the following: platelet count, hemoglobin, fibrinogen, prothrombin, factor V, factor VII, factor VIII, factor IX, factor X, factor XI, factor XII, factor XIII, von Willebrand factor, antithrombin, protein C, protein S, tissue factor pathway inhibitor, PAI-1, PAI-2, thrombin activatable fibrinolysis inhibitor, PT, aPTT, D-dimer, plasminogen, plasmin, thrombin generation parameters, TEG parameters, ROTEM parameters, fibrin clot lysis time parameters, and plasmin generation parameters.
Outcome variables
Primary outcome
PPH in general, as defined in respective studies.
Secondary outcomes
Severe PPH, as defined within respective studies.
Subgroups defined based on the mode of delivery
Vaginal delivery and C-section (elective or emergency C-section).
Study population
The aggregated study cohort comprised pregnant women from whom samples were collected before delivery for the measurement of at least 1 hemostatic biomarker and evaluated for PPH or severe PPH, irrespective of the delivery mode.
If feasible, the descriptive analysis was planned to be stratified based on the mode of delivery.
Data synthesis and statistical methods
In addition to presenting all included studies qualitatively, we conducted quantitative syntheses of studies reporting on the same hemostatic biomarkers. To estimate differences in predelivery hemostatic biomarker levels between women with PPH or severe PPH and controls, we calculated the mean difference (MD) with a 95% CI, fitting a random-effects (RE) meta-analysis. We used standardized MD for PT because studies reporting on PT used different units (ie, percentages or seconds). The amount of between-study variance, τ2, was estimated using the restricted maximum-likelihood estimator. In addition, we quantified the between-study heterogeneity by providing the I2 statistics and calculated 95% prediction intervals, if τ2 was estimated to be nonzero. Tests and confidence intervals were computed using the Knapp and Hartung method.6 Missing means and SDs were calculated from quartiles, if reported, using the method developed by Luo et al.7 Results from the RE models were presented graphically with forest plots. All statistical analyses were performed using R version 4.2.2 (R Core Team 2022, Foundation for Statistical Computing, Vienna, Austria) and the metafor package (version 3.8.1).
A sensitivity analysis was also performed including only the studies that reported PPH or severe PPH outcome based on the definitions per the World Health Organization (WHO).
Results
Selection process and characteristics of included studies
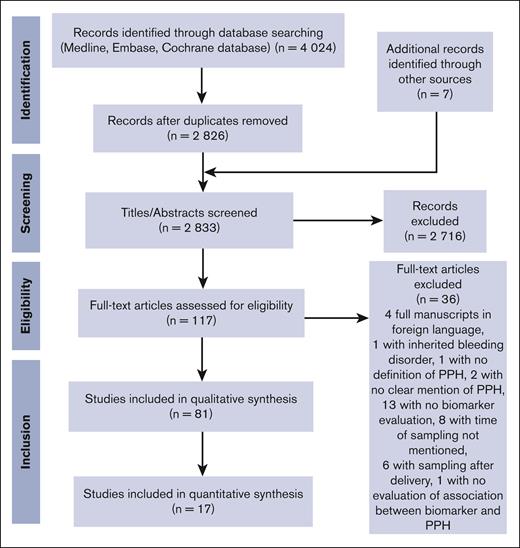
A search of the 3 electronic databases on 18 October 2022 yielded 4024 articles (Figure 1). After removal of the duplicates, C.d.M. and D.M. screened all titles and abstracts available in English (n = 2826), which led to the exclusion of 2716 further articles. The 2 researchers read then the remaining 117 full-text articles and excluded 36 more articles, which led to the final inclusion of 81 articles in the qualitative synthesis.8-88
Because of missing data on reported mean values ± SD or median values for a range of predelivery hemostatic biomarkers in women with PPH/severe PPH and in controls, only 17 articles were finally included in the quantitative synthesis. Table 1 displays the general characteristics of the studies included in the qualitative and quantitative syntheses.
Qualitative synthesis
A total of 81 articles were analyzed, corresponding to 20 317 287 pregnant women, with 94 089 PPH cases and 205 096 severe PPH cases (Table 1). The majority of studies were conducted in Asia (37 studies; 19 408 954 women), followed by Europe (21 studies; 219 215 women) and North America (10 studies; 271 588 women). The African continent was underrepresented, with only 5538 women included in 10 studies. The most frequent study design was on monocenter cohorts (50 studies). The population of pregnant women was unselected in most of the studies, although 35 studies restricted inclusion to singleton pregnancies. Sixty-nine studies reported on vaginal deliveries and 59 on C-sections. Among hemostatic and hematologic biomarkers, hemoglobin was the most frequently studied (55 studies), followed by platelets (33 studies), fibrinogen (14 studies), and PT (10 studies) (Table 1).
Supplemental Tables 2-4 summarize the characteristics of these 81 studies as well as the characteristics of the included women, the pregnancies, and the deliveries.
Hemostatic biomarkers were measured mainly during the third trimester of pregnancy (44 studies), and within 72 hours before delivery in 30 studies. Four studies reported on hemostatic biomarkers that were measured only during the first trimester of pregnancy.22,54,68,79
Characteristics of pregnant women differed notably by the geographical location of the study. For example, obesity was more prevalent among American women than among Chinese women, and stillbirth was more frequent among African women than among European women.
PPH and severe PPH definitions were also quite heterogenous between studies, despite the existence of the definitions by WHO published in 2012.89 As defined by the WHO, PPH is described as a blood loss of ≥500 mL within the first 24 hours after delivery, irrespective of the delivery mode, and severe PPH is defined as a blood loss of ≥1000 mL within the first 24 hours after delivery. These 2 definitions were used in only 24 studies.12-14,16,21,29,31,37,40-42,47,48,50,53,60-62,64,68,69,72,74,80 Ten studies used a definition of PPH that differentiates the amount of blood loss based on the mode of delivery (≥500 mL within the first 24 hours after vaginal delivery and ≥1000 mL after C-section). The other studies either chose other cutoffs for blood loss or used combined criteria, particularly the need for blood transfusion in addition to the amount of blood loss at delivery for severe PPH (supplemental Table 4).
Quantitative synthesis
Overall quantitative synthesis
In total, 17 articles were analyzed, corresponding to 26 654 pregnant women, with 3746 cases of PPH and 1167 cases of severe PPH.8,11,12,14,18,24,26,38,40,42,53,57,58,61,69,71,85 The characteristics of the women are provided in Table 1.
The included population of pregnant women differed notably from the total population included in the qualitative synthesis. Women were mainly from Europe (8 studies, 15 772 women), mostly had singletons (9 studies, 14 622 women), and mostly delivered vaginally (15 studies, 21 417 women). The most-studied hemostatic biomarker was platelets (11 studies), followed by fibrinogen and hemoglobin (9 studies each).
The overall heterogeneity between studies was considerable, as evidenced by wide 95% prediction intervals and I2 values up to 95% (Figures 2-4); supplemental Figure 5.
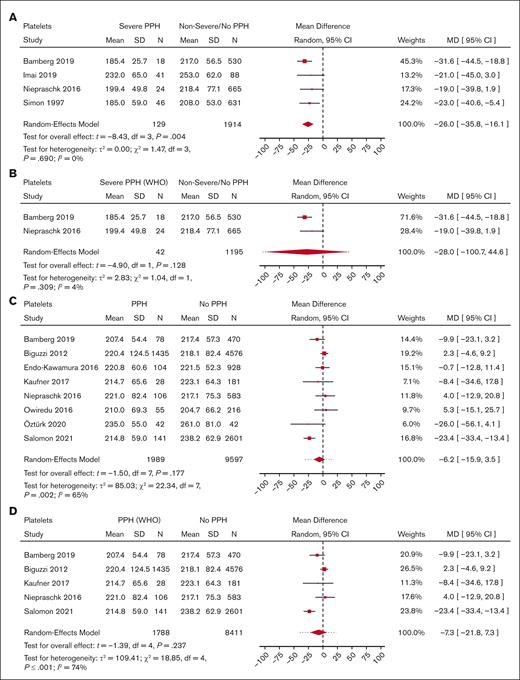
Meta-analysis for predelivery platelet count and its association with severe PPH/PPH. (A) Forest plot of the MD with a 95% CI for predelivery platelet count between women with severe PPH and controls. (B) Forest plot of the MD with a 95% CI for predelivery platelet count between women with severe PPH (per WHO definition only) and controls. (C) Forest plot of the MD with a 95% CI for predelivery platelet count between women with PPH and controls. (D) Forest plot of the MD with a 95% CI for predelivery platelet count between women with PPH (WHO definition only) and controls.
Meta-analysis for predelivery platelet count and its association with severe PPH/PPH. (A) Forest plot of the MD with a 95% CI for predelivery platelet count between women with severe PPH and controls. (B) Forest plot of the MD with a 95% CI for predelivery platelet count between women with severe PPH (per WHO definition only) and controls. (C) Forest plot of the MD with a 95% CI for predelivery platelet count between women with PPH and controls. (D) Forest plot of the MD with a 95% CI for predelivery platelet count between women with PPH (WHO definition only) and controls.
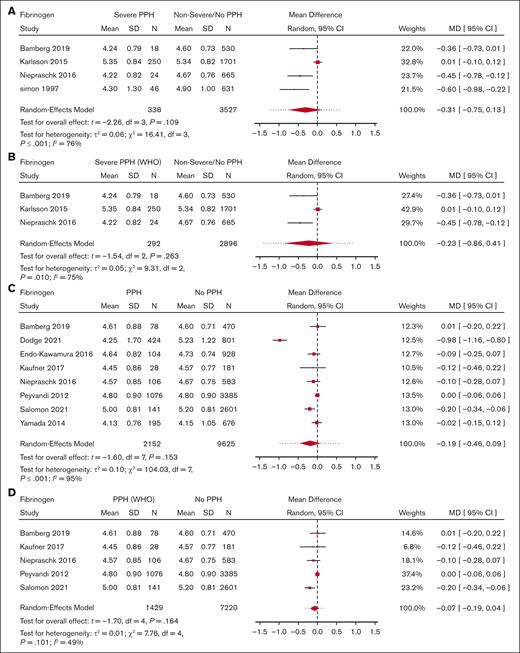
Meta-analysis for fibrinogen level and its association with severe PPH/PPH. (A) Forest plot of the MD with a 95% CI for predelivery fibrinogen concentration between women with severe PPH and controls. (B) Forest plot of the MD with a 95% CI for predelivery fibrinogen concentration between women with severe PPH (WHO definition only) and controls. (C) Forest plot of the MD with a 95% CI for predelivery fibrinogen concentration between women with PPH and controls. (D) Forest plot of the MD with a 95% CI for predelivery fibrinogen concentration between women with PPH (WHO definition only) and controls.
Meta-analysis for fibrinogen level and its association with severe PPH/PPH. (A) Forest plot of the MD with a 95% CI for predelivery fibrinogen concentration between women with severe PPH and controls. (B) Forest plot of the MD with a 95% CI for predelivery fibrinogen concentration between women with severe PPH (WHO definition only) and controls. (C) Forest plot of the MD with a 95% CI for predelivery fibrinogen concentration between women with PPH and controls. (D) Forest plot of the MD with a 95% CI for predelivery fibrinogen concentration between women with PPH (WHO definition only) and controls.
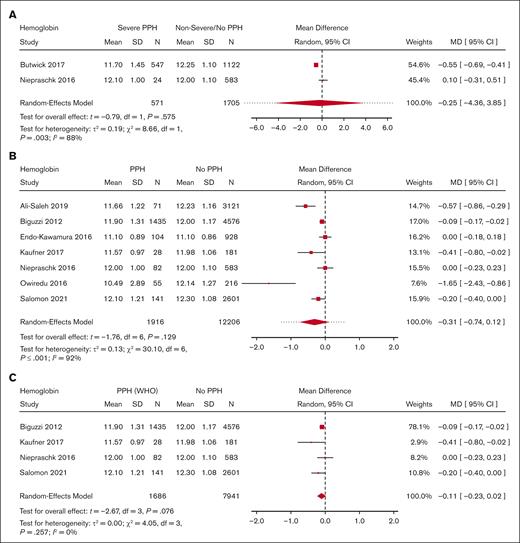
Meta-analysis for predelivery hemoglobin level and its association with severe PPH/PPH. (A) Forest plot of the MD with a 95% CI for predelivery hemoglobin level between women with severe PPH and controls. (B) Forest plot of the MD with a 95% CI for predelivery hemoglobin level between women with PPH and controls. (C) Forest plot of the MD with a 95% CI for predelivery hemoglobin level between women with PPH (per WHO definition only) and controls.
Meta-analysis for predelivery hemoglobin level and its association with severe PPH/PPH. (A) Forest plot of the MD with a 95% CI for predelivery hemoglobin level between women with severe PPH and controls. (B) Forest plot of the MD with a 95% CI for predelivery hemoglobin level between women with PPH and controls. (C) Forest plot of the MD with a 95% CI for predelivery hemoglobin level between women with PPH (per WHO definition only) and controls.
With regard to PPH in general, none of the biomarkers (Figure 2 for platelets, Figure 3 for fibrinogen, and Figure 4 for hemoglobin; supplemental Figure 5 for D-dimer, aPTT, and PT) showed a statistically significant association.
Notably, only 2 studies were included in the analysis for D-dimer, resulting in a large 95% CI of the MD between the PPH group and control group.
Regarding severe PPH, included studies only reported on platelets, fibrinogen, hemoglobin, and factor XIII.
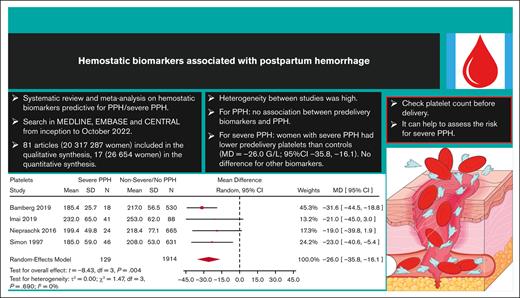
Women who developed severe PPH had, on average, a lower predelivery platelet count than controls (Figure 2) (MD = −26.0 109/L; 95% CI, −35.8 to −16.1; 4 studies). Interestingly, heterogeneity was low between the 4 studies reporting on severe PPH and platelets.
Moreover, there was no significant difference in predelivery fibrinogen concentration or hemoglobin level between women with severe PPH and controls (MD = −0.31 g/L; 95% CI, −0.75 to −0.13; 4 studies for fibrinogen [Figure 3]; MD = −0.25 g/dL; 95% CI, −4.36 to 3.85; 2 studies for hemoglobin [Figure 4]).
The average MD for factor XIII activity between women with severe PPH and controls did not differ significantly from 0 (MD = −0.07 IU/mL; 95% CI, −0.17 to 0.04; 2 studies; supplemental Figure 6).
Quantitative synthesis only with studies using WHO’s definition for PPH
A sensitivity analysis was also performed including only the 7 studies that reported PPH or severe PPH outcome based on WHO’s definitions.12,14,40,42,53,61,69 This specific quantitative synthesis reported on 16 611 exclusively European pregnant women, mainly from monocenter cohort studies of singleton term pregnancies with vaginal delivery.
With regard to PPH in general, none of the biomarkers (Figure 2 for platelets, Figure 3 for fibrinogen, and Figure 4 for hemoglobin; supplemental Figure 5 for aPTT and PT) showed a statistically significant association. With regard to severe PPH, similarly, none of the biomarkers (Figure 2 for platelets and Figure 3 for fibrinogen; supplemental Figure 6 for factor XIII activity) showed a statistically significant association. In particular, predelivery platelet count was not significantly associated with severe PPH in the 2 studies included in this restricted meta-analysis.
Mode of delivery
Because of the small number of women who delivered via C-section (5862 of 26 654 pregnant women), which was reported only in 9 of 17 studies, we chose not to stratify the analysis based on the mode of delivery.
Risk of bias assessment
Risk of bias was evaluated independently by C.d.M. and D.M. and separately for case-control studies (n = 18) and for cohort studies (n = 63) with the Newcastle-Ottawa quality assessment scale.
Following this scale and taking into account an arbitrary cutoff of 6 stars for ranking the studies as being at low (>6 stars) or high (≤6 stars) risk of bias, 47 studies of 81 were considered to be at low risk of bias (supplemental Table 5). These 47 studies comprised 10 case-control studies and 37 cohort studies.
Among the studies at low risk of bias, 11 studies were at very low risk of bias, with a maximum ranking of 9 stars, corresponding to 1 case-control study34 and 10 cohort studies.12,14,19,41,42,44,52,61,62,69 Five of 11 studies at very low risk of bias were analyzed in the quantitative synthesis.12,14,42,61,69
In all studies, the 2 most important factors for bias were in the description of nonresponse rate in case-control studies and in the description of follow-up in cohort studies (supplemental Table 5).
Discussion
In this systematic review of >20 million pregnant women in 81 studies, we aimed to summarize the current literature on the association between predelivery hemostatic biomarkers and PPH and severe PPH.
Our first finding is that between-study heterogeneity on the specific topic of PPH was substantial. For our purpose, we had to pool different populations of pregnant women from cohorts of different sizes and from different settings, who received different monitoring during pregnancy and different management of delivery. Most of all, the definitions of PPH and severe PPH differed between studies, which was also pointed out previously by Dahlke et al when comparing 4 national guidelines on the prevention and management of PPH.90 This heterogeneity among studies made the evaluation of the association between a given predelivery hemostatic biomarker and PPH particularly difficult, and it might explain, at least in part, why platelet count is the only biomarker that was shown to be significantly associated with severe PPH in our RE meta-analysis. We also faced an overrepresentation of Asian women in the qualitative synthesis, and of European women in the quantitative synthesis. Conversely, there was an underrepresentation of African women in this analysis.
Our quantitative synthesis included only 17 studies because of a lack of reported summary statistics of hemostatic biomarkers in the published studies. We chose to pool MDs of hemostatic biomarker values between PPH or severe PPH and controls in our quantitative syntheses instead of adjusted odds ratios (aORs) for PPH and severe PPH: firstly, because aORs were less reported; and secondly, because the cutoff values for each hemostatic biomarker differed among the various studies. Thus, our quantitative analysis did not take into account the numerous confounding factors associated with PPH and severe PPH, which were probably differently distributed among the heterogenous populations of pregnant women. We performed RE meta-analyses with these MDs, using the Knapp-Hartung method, which is a method based on t distributions, thereby providing confidence intervals closer to nominal performance when the number of studies is small. As a consequence, our confidence intervals were large, and we lacked the power to determine differences in the mean values of those hemostatic parameters that had been evaluated in only a few studies, especially D-dimer or FXIII. We did not demonstrate any significant difference in the mean values of fibrinogen concentration or hemoglobin level between severe PPH and controls but the number of studies we analyzed was small. In the literature, conflicting data exist on the association of predelivery fibrinogen concentration or hemoglobin level and severe PPH, which explains that these biological parameters are not used routinely to predict severe PPH. However, we need to interpret the results of our meta-analyses with caution. A lack of statistical significance in the MDs of a given hemostatic biomarker between PPH or severe PPH groups and controls does not rule out a potential use of this biomarker for predicting PPH or severe PPH. The conclusions we can make from these meta-analyses are indeed limited because of substantial between-study heterogeneity and confounding factors.
Our second finding is that, on average, predelivery platelet count was lower in women who developed severe PPH compared with controls, suggesting the potential usefulness of this simple biomarker for predicting severe PPH. Platelets are essential for clot formation and for control of hemorrhage. Platelet count decreases progressively during physiological pregnancy until delivery.91 Thrombocytopenia, defined as a platelet count of <150 109/L, is frequent during pregnancy and can have different causes, mainly gestational thrombocytopenia (the most frequent cause [75%]) but also thrombotic microangiopathy related to preeclampsia (ie, HELLP syndrome) or immune thrombocytopenic purpura (3%). These 2 latter causes were excluded from this study. Infrequently, thrombocytopenia can be related to dramatic pregnancy complications such as acute placental abruption with stillbirth or catastrophic amniotic fluid embolism, causing PPH through resulting disseminated intravascular coagulation. Among the 33 studies included in the qualitative synthesis that reported on predelivery platelet count, 14 documented an association between predelivery platelet count and PPH in multivariable analysis (4 for severe PPH and 11 for PPH in general), with aORs ranging from 1.34 to 19.70 for severe PPH, and from 1.04 to 4.70 for PPH in general. However, platelets cutoffs differed between studies, so we could not conclude from these aggregated data that predelivery thrombocytopenia is associated with PPH or possibly predictive of severe PPH. Furthermore, when we restricted the quantitative synthesis to only studies that used the WHO’s definition for PPH and severe PPH, predelivery platelet count was not significantly associated with severe PPH. However, there was an important loss of power in this analysis because of a reduction in the number of studies. When performing this quantitative synthesis without the Knapp and Hartung method (data not shown), narrower confidence intervals were found, and predelivery platelet count was still significantly associated with severe PPH. However, the Knapp and Hartung method better reflects the uncertainty when trying to perform an RE meta-analysis with a very low number of studies. Interestingly, American obstetric guidelines have incorporated predelivery platelet count in the risk assessment for PPH before delivery, and 3 American risk-assessment tools based on experts’ opinion (New-York Safety Bundle for Obstetric Hemorrhage [NYSBOH], Association of Women’s Health, Obstetric and Neonatal Nurses, and California Maternal Quality Care Collaborative scales) consider a low predelivery platelet count to be a high risk factor for PPH.92
Despite these limitations, this is a unique systematic review on a broad range of predelivery hemostatic biomarkers and their association with PPH or severe PPH. Three systematic reviews and meta-analyses previously analyzed the association between predelivery hemoglobin and PPH.93-95 Of these 3 studies, 2 reported an association between severe anemia and PPH, the main limitation being that severe anemia was not uniformly defined, with reported values ranging from <7 g/dL to <8 g/dL. Surprisingly, no systematic review reported on the association between predelivery platelet count or fibrinogen concentration and PPH or severe PPH. Finally, 3 reviews (1 systematic and 2 nonsystematic), which examined the utility of viscoelastic hemostatic assays (TEG, ROTEM, Sonoclot, Quantra, ClotPro) to predict PPH before delivery, were inconclusive because of a lack of large studies focusing on this specific issue.96-98 Indeed, most studies evaluated viscoelastic assays as a guide for transfusion and plasma replacement during ongoing PPH but not before delivery as a predictive tool for PPH.
There is still a knowledge gap in the prediction and prevention of PPH. Identification of hemostatic biomarkers predictive of PPH or severe PPH could improve peripartum management, for example, by integrating them into a predictive score for PPH, which would be calculated before delivery and would guide treatment decisions (eg, prophylactic tranexamic acid administration or oxytocin use during labor).
Conclusions
In this systematic review and meta-analysis, available data on predelivery hemostatic biomarkers potentially associated with PPH in general and severe PPH were summarized. On average, predelivery platelet count was lower in women who developed severe PPH than that in controls, suggesting the potential usefulness of this simple biomarker for predicting severe PPH. Less-clear evidence for an association with PPH was found for other hemostatic biomarkers. There is a need for further prospective studies using standardized definition of PPH, standardized cutoffs for biological parameters, and a standardized time for blood collection to improve the knowledge on this important issue for pregnant women.
Acknowledgment
The authors thank Tanja Altreiter for proofreading this manuscript.
Authorship
Contribution: C.d.M., I.P., S.N., D.K., and D.M. designed the study; C.d.M., I.P., and S.N. wrote the protocol of the systematic review; C.d.M. and D.M. performed the literature search, made the selection of included studies, and evaluated the risk of bias for each included study; D.K. performed the statistical analyses; C.d.M. wrote the manuscript; and all authors reviewed the manuscript and approved its final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The permanent affiliation for C.d.M. is Internal Medicine, Vascular Medicine and Pneumology Department, UMR 1304, GETBO, UBO, Brest University Hospital, Brest, France.
Correspondence: Claire de Moreuil, Clinical Division of Haematology and Haemostaseology, Department of Medicine I, Medical University of Vienna, Vienna, Austria; e-mail: claire.demoreuil@chu-brest.fr.
References
Author notes
The data that support the findings of this study are available on request from the corresponding author, Claire de Moreuil (claire.demoreuil@chu-brest.fr).
The full-text version of this article contains a data supplement.