TO THE EDITOR:
Cancer health disparities are differences in health outcomes observed across population groups, including patients with acute myeloid leukemia (AML), characterized by various social factors such as race, ethnicity, education, or environmental exposure.1-5 Patients living in areas characterized by higher social deprivation (SD) experience worse health outcomes because of public and private disinvestment, leading to exposure to various health-adverse factors.6,7 Notably, SD is an extrinsic factor that can be changed by measures taken at the community level, and/or overcome by individuals. To our knowledge, no large study of adult AML has hitherto evaluated the impact of SD within large, multicenter clinical trials conducted with standardized treatments to reduce confounding bias owing to health care access.
We analyzed 1893 adults diagnosed with de novo AML (except acute promyelocytic leukemia), including 1233 patients aged <60 years and 660 aged ≥60 years, who were treated on the Cancer and Leukemia Group B/Alliance for Clinical Trials in Oncology frontline treatment protocols (supplemental Figure 1). All patients provided written informed consent to participate in treatment studies, of which protocols were in accordance with the Declaration of Helsinki and approved by institutional review boards at each center.
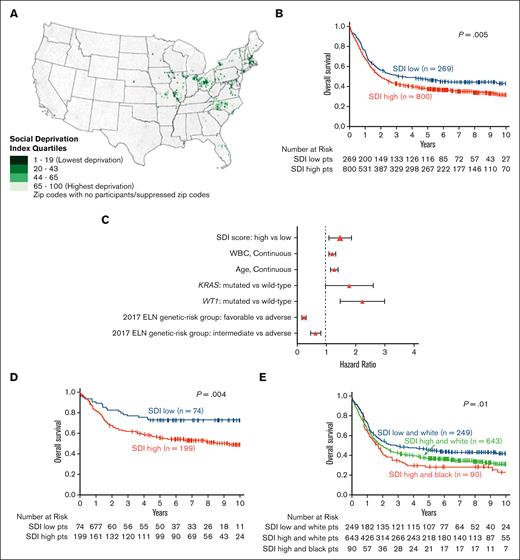
We calculated zip code–level social deprivation index (SDI), a well-validated metric of zip code SD6 (supplemental Data). We divided patients with AML into quartiles based on SDI (trend test, P = .81) and then defined 2 SDI groups: low (patients in quartile 1) and high (patients in quartiles 2-4). Low SDI comprised the lowest 25% of SDI scores and included 323 younger patients and 172 patients aged ≥60 years. High SDI included the upper 75% of SDI scores and included 910 younger and 488 older patients. Figure 1A shows the geographic distribution of patients’ residential zip code SDI.
Geographic distribution and treatment outcomes of adults with AML with respect to SDI assignments, race, and genetic-risk profile. (A) Map of the United States with light-green to dark-green color scale indicating the calculated SDI based on patients’ home zip code. Dark color corresponds to lower SDI score. (B) OS of patients with AML aged <60 years with respect to their SDI grouping (high vs low). (C) Forest plot depicting final multivariable model for OS. (D) OS of patients with AML belonging to the favorable genetic-risk group of the 2017 European LeukemiaNet classification, comparing patients with high SDI with those with low SDI. (E) OS of high- vs low-SDI groups of patients with AML with respect to the patients’ self-reported race. Only 3 Black patients belonged to the low-SDI group and are not depicted in the graphic.
Geographic distribution and treatment outcomes of adults with AML with respect to SDI assignments, race, and genetic-risk profile. (A) Map of the United States with light-green to dark-green color scale indicating the calculated SDI based on patients’ home zip code. Dark color corresponds to lower SDI score. (B) OS of patients with AML aged <60 years with respect to their SDI grouping (high vs low). (C) Forest plot depicting final multivariable model for OS. (D) OS of patients with AML belonging to the favorable genetic-risk group of the 2017 European LeukemiaNet classification, comparing patients with high SDI with those with low SDI. (E) OS of high- vs low-SDI groups of patients with AML with respect to the patients’ self-reported race. Only 3 Black patients belonged to the low-SDI group and are not depicted in the graphic.
The mutational status of 80 protein-coding genes was retrospectively determined centrally at The Ohio State University via companion protocol CALGB 20202.8-10 Experimental details are provided in the supplemental Data.
The baseline characteristics of patients with high and low SDI scores are shown in supplemental Table 1. Most clinical characteristics did not differ significantly between the high- and low-SDI groups. However, Black patients were more frequent in the high-SDI group (9% vs 1%, P < .001).
Among patients aged <60 years, those in the high-SDI group had lower percentages of BCOR (3% vs 7%, P = .03), IDH1 (6% vs 11%, P = .03), and STAG2 (2% vs 5%, P = .02) mutations, of mutations in genes belonging to the cohesin complex (11% vs 18%, P = .03) and methylation-related (36% vs 46%, P = .03) groups, and a higher percentage of mutations in genes encoding tumor suppressors (18% vs 11%, P = .02) than patients in the low-SDI group, suggesting differences in the disease biology (supplemental Tables 2 and 3).
Younger patients in the high-SDI group had shorter disease-free survival (DFS; median, 1.5 vs 2.8 years, P = .02) and overall survival (OS; median, 1.9 vs 3.0 years; P = .005), and more of them died during follow-up (65% vs 55%, P = .004) compared with younger patients the low-SDI group (Table 1; supplemental Figures 1A-B). Notably, fewer patients residing in the high SDI areas underwent off-protocol hematopoietic stem cell transplantation in first complete remission (CR) than patients in low SDI areas (12% vs 17%, P = .04).
In multivariable analyses, high SDI score associated independently with shorter OS both in the entire cohort of younger patients (hazard ratio [HR], 1.28; 95% confidence interval [CI], 1.06-1.55; P = .001) and in the subset of younger patients with available molecular information (HR, 1.43; 95% CI, 1.10-1.86; P = .008; Figure 1C; supplemental Table 4). However, because both BCOR11-13 and IDH111,14 mutations have been reported to adversely affect prognosis of younger adults with AML, the lower frequency of these mutations in high SDI–group patients, whose outcomes are worse, makes it less likely that the observed survival disparities can be explained by underlying biology of the disease.
Importantly, there were no significant differences in CR or relapse rates between SDI groups (Table 1), suggesting that response to induction was not inherently different. There was also no significant difference in early death rate, indicating that a delay in diagnosis and/or starting therapy might not be a factor driving differences in survival outcomes. Similarly, there was no difference in the number of consolidation cycles received that would support differences in post-CR consolidation intensity.
Notably, high SDI score, compared with low SDI score, was associated with shorter DFS (5-year rates: 48% vs 60%; P = .05; supplemental Figure 2B) and OS (5-year rates: 56% vs 73%; P = .004; Figure 1D) in patients belonging to the 2017 European LeukemiaNet15 favorable-risk group (supplemental Table 5). No significant impact of SDI scores on DFS or OS was seen in patients belonging to either the 2017 European LeukemiaNet intermediate-risk or adverse-risk groups (supplemental Figure 3). The association of SD with outcomes within the favorable genetic-risk group is especially concerning, because patients in this genetic-risk group have the highest likelihood of being cured with chemotherapy alone. Because these patients are generally not offered allogeneic hematopoietic stem cell transplantation in first CR, transplant availability does not seem to have played a major role in the observed survival disparities. In contrast, the higher percentage of patients in the high-SDI group who died during follow-up, may suggest that impediments to, or delay of, care and support after completion of protocol treatment might have contributed to survival disparities.
Given the previously reported shorter survival of non-Hispanic Black patients with AML,16,17 we analyzed the survival of Black and White patients separately with respect to the assigned SDI group. In White patients, belonging to the high-SDI group (compared with those in the low-SDI group) was associated with shorter DFS (median: 1.5 years vs 2.3 years; P = .04; supplemental Figure 2C) and OS (median: 2.0 vs 2.9 years; P = .01; Figure 1E and supplemental Table 6), whereas no direct analysis of the impact of SDI could be made in Black patients because of the very low number (n = 3) of Black patients in the low-SDI group. However, Black patients belonging to the high-SDI group had comparable DFS (median: 1.0 vs 1.5 years; P = .45) and OS (median: 1.7 vs 2.0 years; P = .27) to those of White patients who lived in areas with high SDI.
In contrast to younger patients, we found no significant differences in the frequency distribution of AML-associated gene mutations (n = 660; supplemental Table 7) or survival between the SDI groups in older patients, both among patients treated with chemotherapy and those receiving treatment with decitabine with or without bortezomib18 (supplemental Table 8). This is not surprising, because older patients are known to have worse outcomes15; thus, any potentially negative impact of SDI would be more difficult to detect in this age group.
Limitations of our study include the fact that we analyzed only patients enrolled on frontline clinical treatment protocols, which means that patients who are disadvantaged by lack of trial access19 were omitted. Representativeness in AML clinical trials has been recently identified as an important contributor to research disparities, including both SD and racial-ethnic identity.20,21 In this study, we used zip codes and Eastern Cooperative Oncology Group performance status as validated surrogates for SDI determination and patient comorbidities, respectively. However, this highlights the need to collect such data prospectively in future clinical trials.
We believe that our results uncover a potentially modifiable risk factor, which, if addressed, might improve outcomes of patients with favorable-risk AML without changes in treatment modality or intensity, and shows the necessity to investigate possible additional risk factors in patients with AML.
Acknowledgments: This study celebrates the life and accomplishments of Clara D. Bloomfield (1942-2020), who died unexpectedly on 1 March 2020. The authors are grateful to the patients who consented to participate in these clinical trials and the families who supported them; to Christopher Manring and the CALGB/Alliance Leukemia Tissue Bank at The Ohio State University Comprehensive Cancer Center, Columbus, OH, for sample processing and storage services; and to Lisa J. Sterling for data management. Research reported in this publication was supported by an allocation of computing resources from The Ohio Supercomputer Center and Shared Resources (Leukemia Tissue Bank). Research reported in this publication was supported, in part, by the National Cancer Institute of the National Institutes of Health under award numbers U10CA180821, U10CA180882, and U24CA196171 (to the Alliance for Clinical Trials in Oncology), UG1CA189824, UG1CA233338, UG1CA233339, P50 CA140158, UG1CA233331, UG1CA233180, R35CA197734, UG1CA189850, UG1CA233327, and 5P30CA016058; the Coleman Leukemia Research Foundation; ASH Junior Faculty Scholar and ASH Bridge award (A.-K.E.); the Leukemia & Lymphoma Society Translational Research Grant (A.-K.E.); The Leukemia Research Foundation (A.-K.E.); the National Comprehensive Cancer Network Foundation Young Investigator Award (J.S.B.); the Alliance for Clinical Trials in Oncology Scholar Award (J.S.B.); The D. Warren Brown Foundation; Pelotonia (A.-K.E. and A.S.M.). Support to Alliance for Clinical Trials in Oncology and Alliance Foundation Trials programs is listed at https://acknowledgments.alliancefound.org. Trial registration numbers are NCT00048958, NCT00899223, and NCT00900224. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contribution: M.R., A.-K.E., and J.J.P. contributed to the study design; M.R., K.M., J.K., K.T.L., C.C.O., J.C.B., E.D.P., J.J.P., and A.-K.E. contributed to the data interpretation; M.R., K.M., J.J.P., and A.-K.E. wrote the manuscript; A.-K.E., A.S.M., J.S.B., and S.O. performed laboratory-based research and genomic data analysis; J.K. and D.N. performed statistical analysis; A.J.C., K.M., W.G.B., B.L.P, G.L.U., R.M.S., R.A.L., and J.C.B. were involved directly or indirectly in the care of patients and/or sample procurement; and all authors read and agreed on the final version of the manuscript.
Conflicts-of-interest disclosure: A.S.M. received research funding from Leukemia and Lymphoma Society’s Beat AML clinical study, Aptevo, Daiichi Sankyo, Glycomemetics, Kartos Pharmaceuticals, Xencor, and Genentech; and is a consultant for Aptevo, AbbVie, BMS, Glycomemetics, Jazz Pharmaceuticals, Kura Oncology, and Syndax Pharmaceuticals. J.S.B. is a consultant for, and reported honoraria from, KITE, INNATE, AstraZeneca, and AbbVie. W.G.B. reported honoraria from AbbVie, Syndax, and AmerisourceBergen; and research funding from Celyad Oncology, Nkarta, Xencor, Forma Therapeutics, and Leukemia and Lymphoma Society. G.L.U. is a consultant for AbbVie, Agios, Jazz, GlaxoSmithKline, Genentech, and Novartis; reported honoraria from Astellas; and research funding from Macrogenics. R.M.S. consults for AbbVie, Agios, Aprea, Arog, Boston Pharmaceuticals, Bristol Myers Squibb, Celgene, Foghorn Therapeutics, GlaxoSmithKline, Innate, Janssen, Jazz, Macrogenics, Novartis, Onconova, and Takeda; received research funding from AbbVie, Agios, Arog, and Novartis; and serves on the advisory committee or board of directors for Actinium, Amgen, Astellas, BerGen Bio, Boston Pharmaceuticals, Elevate Bio, Gemoab, Syndax, Syntrix/ACI, and Syros. R.A.L. received research funding from Astellas, Cellectis, Gilead, Novartis, and Rafael Pharmaceuticals; and consults for AbbVie, CVS/Caremark, Epizyme, and Takeda. J.C.B. consults for Astellas, AstraZeneca, Novartis, Pharmacyclics, Syndax, and Trillium; receives honoraria from Astellas, AstraZeneca, Novartis, Pharmacyclics, Syndax, and Trillium; is a chairman of the scientific advisory board of Vincerx Pharmaceuticals and a member of the advisory committee of Newave; and is a current equity holder of Vincerx Pharmaceuticals. E.D.P. received research funding from Merck and Pfizer. A.-K.E. is a spouse of Christopher J. Walker who is currently employed by Karyopharm Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Ann-Kathrin Eisfeld, The Ohio State University Comprehensive Cancer Center, 460 West 12th Ave, Room 850, Columbus, OH 43210-1228; e-mail: Ann-Kathrin.Eisfeld@osumc.edu; Jessica Kohlschmidt, The Ohio State University Comprehensive Cancer Center, OSU Medical Center, Room 353, 650 Ackerman Rd, Columbus, OH 43202-4500; e-mail: Jessica.Kohlschmidt@osumc.edu; Krzysztof Mrózek, The Ohio State University Comprehensive Cancer Center, OSU Medical Center, Room 351, 650 Ackerman Rd, Columbus, OH 43202-4500; e-mail: Krzysztof.Mrozek@osumc.edu; and Jesse J. Plascak, Division of Cancer Prevention and Control, Department of Internal Medicine, College of Medicine, The Ohio State University, 1590 N High St, Ste 525, Columbus, OH 4320; e-mail: Jesse.Plascak@osumc.edu.
References
Author notes
∗J.J.P. and A.-K.E. are the senior authors who contributed equally to the work.
Data are available on request from the corresponding author, Ann-Kathrin Eisfeld (Ann-Kathrin.Eisfeld@osumc.edu).
Presented, in part, at the 63rd annual meeting of the American Society of Hematology, Atlanta, GA, 11 to 14 December 2021.
The full-text version of this article contains a data supplement.