Key Points
The use of statins was associated with a significant decrease in the risk of MPNs.
A dose-response relationship supported the association with increasing treatment duration, in particular ≥5 years.
Abstract
Previous studies have indicated a possible cancer-protective effect of statins in solid cancers; however, this has never been investigated in myeloproliferative neoplasms (MPNs). We aimed to investigate the association between statin use and the risk of MPNs in a nested nationwide case-control study, using Danish national population registries. Information on statin use was obtained from the Danish National Prescription Registry, and patients diagnosed with MPNs between 2010 and 2018 were identified from the Danish National Chronic Myeloid Neoplasia Registry. The association between statin use and MPNs was estimated using age- and sex-adjusted odds ratios (ORs) and fully adjusted ORs (aORs), adjusting for prespecified confounders. The study population included 3816 cases with MPNs and 19 080 population controls (5:1) matched for age and sex using incidence density sampling. Overall, 34.9% of the cases and 33.5% of the controls ever used statins, resulting in an OR for MPN of 1.07 (95% confidence interval [CI], 0.99-1.16) and an aOR of 0.87 (95% CI, 0.80-0.96), respectively. 17.2% were categorized as long-term users (≥5 years) among the cases compared with 19.0% among controls, yielding an OR for MPN of 0.90 (95% CI, 0.81-1.00) and an aOR of 0.72 (95% CI, 0.64-0.81). Analysis of the effect of the cumulative duration of statin use revealed a dose-dependent response, and the association was consistent for sex, age, and MPN subgroups and across different statin types. Statin users were associated with significantly lower odds of being diagnosed with an MPN, indicating a possible cancer-preventive effect of statins. The retrospective design of this study precludes causal inferences.
Introduction
Statins are a group of pharmaceutical drugs with inhibitory effects on the 3-hydroxy-3-methylglutaryl–coenzyme A reductase and are used as cholesterol lowering agents for primary and secondary prevention of cardiovascular diseases.1 The use of statins has increased dramatically over the past decades because of increasing incidences of lifestyle-related diseases, leading to them being listed among the most prescribed drugs.2
The classic Philadelphia chromosome–negative myeloproliferative neoplasms (MPNs) are clonal neoplastic diseases that include essential thrombocythemia (ET), polycythemia vera (PV), myelofibrosis (MF), and MPN-unclassifiable (MPN-U). MPNs are characterized based on myeloproliferation and abnormal peripheral blood counts, increased risk of vascular complications, organomegaly, hyperinflammation, constitutional symptoms, and a propensity to develop into acute myeloid leukemia. In the advanced MF stage, progressive bone marrow fibrosis may exacerbate peripheral blood counts, organomegaly, and constitutional symptoms. Constitutive activation of the JAK-STAT pathway via recurrent somatic mutations in JAK2 V617F, CALR, or MPL is a hallmark of MPNs. Increased activity in the JAK-STAT pathway is also associated with hyperinflammation and bone marrow fibrosis formation by altering the bone marrow microenvironment.3-6
Preclinical studies of statins have shown antileukemic activity in myeloid neoplasms.7-9 The mechanisms by which statins contribute to reduced cell viability and proliferative activity have not been fully elucidated. However, several effects, including antiproliferative, proapoptotic, antiangiogenic, and anti-inflammatory effects together with the decreased release of several growth factors support the notion that statins may have the potential to prevent the development of fibrotic and neoplastic diseases.10,11 Knowledge regarding the pleiotropic and preventive effects of statins is of clinical and great public health importance. This study aims to investigate whether statin use affects the risk of developing MPNs in the Danish population.
Materials and methods
Study design and setting
We conducted a population-based nested case-control study, comparing statin use among patients diagnosed with MPNs between 2010 and 2018 with that of a matched MPN-free population. The Danish population has free access to medical care provided by a tax-supported public health care system, including general practitioners and hospital services. Patients with suspected MPNs are referred to a public hospital clinic for nationally standardized diagnostic workup and treatment.
Nationwide Danish registers
The unique 10-digit personal identification number (Det Centrale Personregister number)12,13 was used to merge 5 nationwide health registers: Danish Civil Registration System,12,13 the National Patient Register (NPR),14,15 the Danish National Chronic Myeloid Neoplasia Registry (DCMR),16 the Danish National Prescription Registry (DNPR),17,18 and the Danish Education Registers19 (more detailed information on the registers is provided in the supplemental Material).
Selection of cases and controls
Cases were identified using DCMR covering >90% of the MPN cases in Denmark since 201016 and were defined per the World Health Organization 2008 (or later) criteria20,21 as Danish residents with an MPN (ET, PV, MF, or MPN-U) diagnosis between 1 January 2010 and 31 December 2018. The index date was defined as the first date of MPN diagnosis per the DCMR data. Identified individuals had to be Danish residents for at least 10 consecutive years before the index date to ensure a minimum of 10 years of follow-up for statin ascertainment. The Danish Civil Registration System was used to select 5 random controls for each case from the Danish general population, matched based on age (birth year), sex, and incidence density sampling.22 Cases and matched controls were excluded if they were previously registered with an MPN diagnosis or other cancers (except nonmelanoma skin cancer and carcinomas in situ, see supplemental Material) before the index date because of the association between previous chemo- or radiotherapy and the development of therapy-related MPNs.
Statin use and exposure assessment
The exposure assessment of statins was identified using the DNPR (Anatomical Therapeutic Chemical Classification System codes: C10AA, C10BA, and C10BX). Ever-use of statins was defined as having redeemed at least 1 prescription of statins before the index date, and never-use was defined as having no filled prescription of statins before the index date. Long-term statin use was defined as ≥5 years of statin treatment. However, because the DNPR lacked information on the calculated cumulative duration of exposure, we estimated the cumulative treatment duration based on every redeemed prescription by the participants, assuming a daily intake of 1 tablet of the prescribed dose per day and added 25% as additional days to account for variations in prescription filling patterns and minor incompliance. To reduce the possibility of reverse causation (ie, increased medical attention around the MPN diagnosis), we disregarded all prescriptions redeemed in the year before the index date of our main analysis.
Covariates and confounders
Using data from the NPR (hospital diagnoses to assess comorbidity), the DNPR (prescriptions related to conditions or use of drug with suggested cancer-protective properties), and the Danish Education Registers (demographics and highest achieved education), we adjusted for a set of prespecified potential confounders: (1) highest achieved educational level (primary school, high school, short/intermediate education, or long education) as a measure of socioeconomic status; (2) Charlson Comorbidity Index23 (0, 1, or ≥2); (3) the use of drugs with suggested cancer-modulating effects,24 including aspirin, nonsteroid anti-inflammatory drugs, alendronate, immunosuppressants, and metformin (≥2 previous prescriptions for all); and (4) markers of diabetes (prescription of antidiabetic drugs and diagnose codes), markers of smoking (diagnosis of chronic obstructive pulmonary disease or use of broncho-dilating inhalation agents containing an antimuscarinic component), markers of autoimmune diseases (ADs) (diagnose codes or prescription of immunomodulating drugs), and markers of extensive alcohol consumption (diagnose codes and prescription of disulfiram). Information to calculate the Charlson Comorbidity Index was collected from the NPR (International Statistical Classification of Diseases and Related Health Problems-10 codes) and DNPR (antidiabetics including insulin) derived from the diagnoses of 19 chronic conditions. Data to assess confounding factors were disregarded 1 year before the index date in the primary analysis. More detailed information, including codes and definitions, is provided in supplemental Table 1.
Secondary and sensitivity analysis
Several prespecified secondary analyses were performed. A stratified analysis based on the subtype of MPN (MF, PV, ET, and MPN-U), sex (male and female), age group (<60 years, 60-75 years, or >75 years), and absence of diabetes and ADs was performed to investigate the association between stratified subsets with long-term statin use and the risk of MPN subtypes. We investigated the effects of the different subtypes of statins by stratifying our analysis into 3 groups, including simvastatin, atorvastatin, and “other statins” (including lovastatin, pravastatin, fluvastatin, and rosuvastatin). Furthermore, for long-term use, we repeated the analyses, adjusting for single potential confounders on the estimated odds ratios (ORs) to show the effect of confounder adjustment. As a sensitivity analysis, we varied the lag-time (the disregarded time before index date when assessing exposure and confounders) for the analysis of long-term use by 6-month increments to investigate the presence of reverse causation.
Statistical analysis
Categorical variables were reported as counts and percentages, whereas continuous variables were reported as medians with interquartile ranges (25th-75th percentile). We assessed the association between statin use (exposure) and MPNs (outcome), using conditional logistic regression to obtain adjusted (for age, sex, and index date) ORs, fully adjusted ORs (aORs; adjusted for the previously mentioned confounders), and corresponding 95% confidence intervals (CIs). Statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc), RStudio version 1.1.447 (RStudio Inc), and R version 3.6.1 (R foundation for Statistical Computing). The study was approved by the Data Responsible Institution (North Denmark Region, approval number: 2021-034) in accordance with the general data protection regulation.
Results
Primary analysis
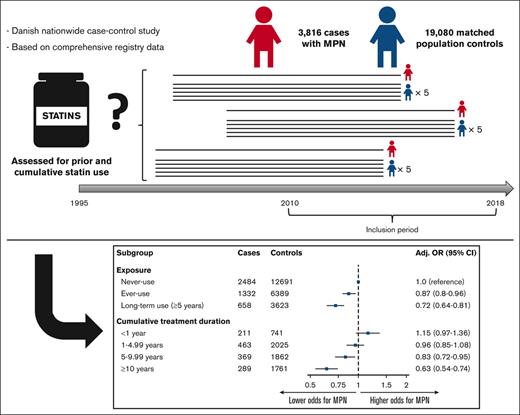
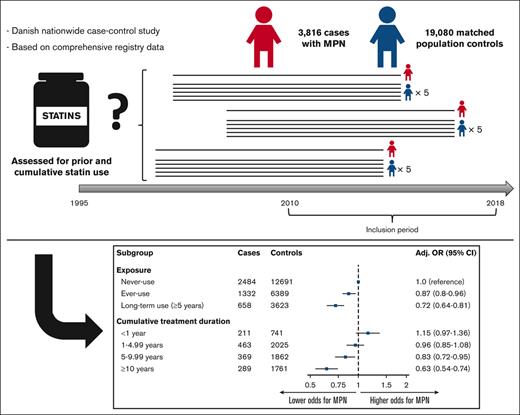
A total of 3816 cases of MPNs registered in the DCMR, including 1306 with PV (34.2%), 1307 with ET (34.3%), 574 with MF (15.0%), and 629 with MPN-U (16.5%) were identified during the surveyed period (Table 1). The cases were matched to 19 080 controls, and the baseline characteristics for both groups are shown in Table 1. Notable differences included higher Charlson Comorbidity Index and higher previous aspirin use among cases (Table 1). The remaining characteristics were well balanced between the cases and controls. Overall, 34.9% of cases and 33.5% of controls were ever-users of statins, resulting in an age- and sex-adjusted OR for MPN of 1.07 (95% CI, 0.99-1.16) (Table 2). 17.2% were categorized as long-term users (>5 years) among the cases compared with 19.0% among controls, yielding an age- and sex-adjusted OR for MPN of 0.90 (95% CI, 0.81-1.00). When adjusting for potential confounders, the aORs for MPN were 0.87 (95% CI, 0.80-0.96) and 0.72 (95% CI, 0.64-0.81) for ever-use and long-term use of statins, respectively. In a dose-response analysis, cumulative treatment duration revealed a clear dose-dependent response, with an insignificant association between short use (<1 year) and an aOR of 1.15 (95% CI, 0.97-1.36) but decreasing aOR with exposure length after 1 year (1-4.99 years: aOR, 0.96 [95% CI, 0.85-1.08]; 5-9.99 years: aOR, 0.83 [95% CI, 0.72-0.95]; and ≥10 years: aOR, 0.63 [95% CI, 0.54-0.74]) (Table 2).
Secondary and sensitivity analyses
In the secondary stratified analysis restricted to long-term use of statins (Table 3), we found comparable estimates across all age groups, with adjusted ORs for ages <60, 60 to 75, and >75 years being 0.80 (95% CI, 0.52-1.25), 0.77 (95% CI, 0.65-0.91), and 0.65 (95% CI, 0.54-0.78), respectively. When stratifying based on sex, we found a strong association with an adjusted OR of 0.57 (95% CI, 0.47-0.68) for males compared with 0.88 (95% CI, 0.75-1.04) for females. This effect was consistent in a subset of subjects with no diabetes and no ADs (Table 3). When investigating subgroups of MPNs, long-term use of statins was associated with decreased odds of MF (aOR, 0.60; 95% CI, 0.44-0.82), PV (aOR, 0.73; 95% CI, 0.59-0.89), and MPN-U (aOR, 0.52; 95% CI, 0.39-0.71), whereas no association was found for ET (aOR, 0.89; 95% CI, 0.72-1.10).
When investigating different statin types, the effect was consistent irrespective of the statin group with aORs for ever-use ranging from 0.72 to 0.86 and for long-term use from 0.65 to 0.72 (supplemental Table 2). For both simvastatin and other statins, a clear dose-response pattern was observed, whereas for atorvastatin, the aORs were similar (0.71-0.73) based on the cumulative treatment duration (supplemental Table 2). The effects of adjustment for single potential confounders are presented in supplemental Table 3. Except for previous aspirin use, univariable adjustment for each confounder had a limited influence on the estimated OR.
In our sensitivity analysis of varying lag time, we found a lower aOR using no lag time of 0.51 (95% CI, 0.45-0.57); however, when stepwise adding 6 months of more lag time (up to 36 months), the analysis led to similar aORs (0.71-0.73; supplemental Table 4).
Discussion
In this Danish nationwide case-control study, statin use was associated with a reduced risk of MPNs, suggesting a possible chemo-preventive effect on cancer. The protective effect was most pronounced for long-term use and was consistent for all statin subtypes. Notably, we found a particularly strong effect among males.
To the best of our knowledge, this is the first study to investigate the association between statin use and risk of MPNs. However, the chemo-preventive effects of statins have been investigated in a variety of other types of cancer. A large Danish population–based observational study, investigating the effect of statin use and cancer-related mortality among 13 patients with nonhematologic malignancies, showed that patients using statins before the cancer diagnosis had lower all-cause mortality and cancer-related mortality compared to patients with cancer who did not use statins.25 Additionally, several large-scale epidemiologic studies have associated the use of statins with reduced risk of pancreatic, hepatic, lung, and gastrointestinal tract cancers.26-28 Only a handful of studies have focused on the association between statin use and hematologic malignancies summarized in meta-analyses.29-31 Overall, statin use was found to be associated with statistically significant reduction in the risk of hematologic malignancies in 2 of 3 meta-analyses in previous observational and prospective studies.29-31 Specifically, the risks of non-Hodgkin lymphoma and leukemia were reduced, whereas no differences were seen for multiple myeloma or Hodgkin lymphoma.29,30 To date, no randomized clinical trial (RCT) has investigated the cancer chemo-preventive effect of statins as the primary objective. However, several large RCTs investigating statins as a secondary prevention for cardiovascular diseases have reported long-term outcomes, including the development of malignancies. Two previous meta-analyses including ≥27 RCTs, comprising >100 000 individuals with an average follow-up of ∼4 to 5 years, found no association between statins and overall cancer risk.32,33 Importantly, it should be noted that RCTs may not be designed to find rare effects occurring after longer induction periods of statins.
The mechanisms underlying the cancer chemo-preventive properties of statins have not yet been fully elucidated. The mevalonate pathway is upregulated in cancers, and an increased mevalonate demand is a hallmark of carcinogenesis. The proposed mechanisms of statins include the downstream effects of inhibiting the 3-hydroxy-3-methylglutaryl–coenzyme A and consequently lowering the levels of mevalonate and associated metabolites, leading to the arrest of tumor initiation and growth.34,35 Interestingly, variants in the gene encoding the 3-hydroxy-3-methylglutaryl–coenzyme A reductase, representing proxies for statin treatment, were shown to reduce the overall cancer risk via a cholesterol-independent pathway.36 Furthermore, evidence also suggests a role of statins in antineoplastic treatment by inhibiting the mevalonate biosynthetic pathway. The inhibition of this pathway is believed to upregulate proapoptotic proteins, leading to increased sensitivity to antineoplastic treatment in hematologic malignancies (ie, BCL2 inhibitors).37 Preclinical studies on human cell lines suggest that JAK/STAT-signaling relies on the cholesterol-dependent integrity of membrane lipid rafts under normophysiological and pathophysiological conditions.38,39 However, the disturbance of the cellular membrane by statin treatment disrupted the pathological JAK2 V617F–mediated signal transduction, whereas this was not observed for wild-type JAK2 activation.39 These findings indicate that pathological JAK/STAT activation may be more reliant on the integrity of the cellular membrane and therefore more sensitive to inhibition by statins.
MPNs are massively undiagnosed, and a Danish study showed that common MPN-driver mutations (JAK2 V617F and CALR) are present in a proportion of the general population at low allele frequencies,40 entailing an increased risk of thrombosis and undiagnosed MPNs.40-42 Speculatively, statin treatment might inhibit clone growth in this pre-MPN phase, equivalent to other premalignant hematologic conditions.43 Highly intriguing, statin use in the general population has been shown to lower the markers of oxidatively generated damage to DNA and RNA. In MPNs, oxidative stress and chronic inflammation44 are likely being elicited by the JAK-STAT activation caused by the JAK2 V617F mutation.45 The rationales and perspectives for using statins in the clonal premalignant stage of MPNs and from the time of MPN diagnosis have most recently been accentuated, putting in perspective that statins may have the potential to lower the JAK2 V617F allelic burden, thereby dampening the chronic inflammatory state, which is considered of importance for clonal expansion and evolution toward overt MPN.42,46 Statin treatment as monotherapy has also been reported to decrease the need of phlebotomies in patients with PV47 and together with bisphosphonate treatment to reduce the need of phlebotomies in concert with a significant reduction in the JAK2 V617F allelic burden.48
The strength of this study is the population-based design–limiting selection bias because all cases with an MPN diagnosis between 2010 and 2018 were included (except those with previous cancers), yielding a high degree of generalizability. The use of DCMR to identify MPN cases offers excellent coverage and a high degree of certainty of diagnosis.16 This study has several limitations in relation to the register-based retrospective design. We recognize that patients receiving statins may differ substantially from those who do not. Studies on statins and cancer have the potential to suffer from healthy user bias; that is, patients with statin use may be more aware of their health and thus improve their health habits, leading to a lower risk of developing cancer, including MPNs.49 Moreover, statin users could also differ in other risk factors associated with decreased or increased risk of cancer, for example, socioeconomic factors or lifestyle. The prescription of statins may also cause temporary or persistent increases in medical surveillance. However, this bias, known as surveillance bias should, if present, result in an upward bias in the effect estimate, resulting in a null estimate or an association with an increased risk of MPNs. Furthermore, statins may be prescribed for a symptom related to MPNs; for example, the diagnosis of a stroke that leads to investigations of underlying causes and risk factors, such as hypercholesterolemia and statin treatment. Hypothetically, stroke could be the first sign of increased thromboembolic risk related to MPNs, leading to protopathic bias, because the event (stroke) could possibly lead to the prescription of a statin and thus reverse causation. To handle this bias, we performed our primary analysis with a lag time for prescription and an assessment of confounding factors for 12 months. Even so, we found no evidence of an increased risk of MPNs with respect to the initiation of statin in our sensitivity analysis of different lengths of lag time. In contrast, we found an even more protective effect in the analysis with no lag time.
We acknowledge the potential risk of confounding, and we cannot exclude that our findings can be explained using residual confounding, that is, familial disposition to MPNs and lifestyle, including smoking and dietary habits. Although we adjusted for several conditions known to influence statin prescription (exposure) and/or the development of MPNs (outcome), the data quality for confounder assessment remains a limitation. Most importantly, we did not have definitive data on smoking habits and anthropometric variables for obesity, which could introduce unmeasured bias if not dealt with. Therefore, the adjustment included proxies for tobacco smoking and being overweight or obese. For example, in this study, <5% of the individuals had a marker of overweight or obesity compared with a recent Danish study showing that the prevalence of obesity increased from 6% in 1987 to more than 18% in 2021.50 This is partly due to the practice of registration in the NPR, in which the coverage of secondary diagnoses is limited; that is, for overweight ∼11%.51 Because overweight or obesity is associated with hypercholesterolemia and cardiovascular disease, it would be associated with statin use; however, overweight and obesity have also been associated with an increased risk of MPN.52 Thus, this misclassification bias would result in an upward bias in the effect estimate and, in our case, underestimating the association between statins and decreased risk of MPNs. Smoking is a known risk factor for developing cardiovascular disease and MPN.53-56 Our data source did not include data on tobacco smoking; hence, the adjustment relied on markers of medication or conditions related to tobacco smoking. Again, residual confounding because of missing data on tobacco smoking would bias our results toward an increased risk of MPN associated with statin use and not an inverse association.
In conclusion, in this Danish nationwide case-control study, we found evidence of a significant association between statin use and a decreased risk of MPNs. Several analyses have supported causality, including a dose-response relationship, and adding to this, a strong biological rationale also supports causality. However, because of the design and retrospective nature of our study, causality cannot be inferred. Our results and the safety and lack of toxicity of statin merit further investigation of statins as protective agents for the development of MPNs and MPN-associated secondary cancers, thrombosis, and inflammation-driven comorbidities. Randomized chemoprevention trials are needed to confirm the causality between statin use and a reduced risk of MPNs.
Acknowledgments
The authors have profound gratitude to everyone who has participated in forming and collecting data for the National Chronic Myeloid Neoplasia Registry, the Danish Clinical Quality Program, and the National Clinical Registries (RKKP) for providing and governing the data.
This study was supported by the Danish Cancer Society.
Authorship
Contribution: D.T.K., A.K.Ø., L.H.K.J., M.T.S., T.C.E.-G., and A.S.R. conceived and designed the study; D.T.K., A.K.Ø., M.T.S., L.H.H., J.S., M.H.H., A.P.V., M.B., H.C.H., and A.S.R. provided the study materials or patients; D.T.K., A.K.Ø., and A.S.R. collected and assembled the data; D.T.K., A.K.Ø., L.H.K.J., M.T.S., T.C.E.-G., and A.S.R. analyzed and interpreted the data; and all authors wrote the manuscript, approved the final version of the manuscript, and are accountable for all aspects of the work.
Conflict-of-interest disclosure: L.H.K.J. received honoraria from Roche. L.H.H. received a research grant from Celgene. H.C.H. is a member on the advisory boards of AOP Orphan and Incyte; is part of the scientific committee of Incyte; and received a research grant from Novartis A/S. T.C.E.-G. declares previous employment with Roche (ended within 24 months) and received the speaker’s fee from AbbVie (April 2021). A.S.R. received honoraria and teaching fees from Pfizer; congress fees from Jazz Pharmaceuticals; and honoraria and consultancy fees from AbbVie. The remaining authors declare no competing financial interests.
Correspondence: Anne Stidsholt Roug, Department of Hematology, Aalborg University Hospital, Hobrovej 18-22, DK-9000 Aalborg, Denmark; e-mail: anne.roug@rn.dk.
References
Author notes
∗D.T.K. and A.K.Ø. contributed equally to this work.
Per Danish law, the data cannot be shared directly; however, they can be accessed through application.
Additional information is available on request from the corresponding author, Anne Stidsholt Roug (anne.roug@rn.dk) or at www.rkkp.dk/in-english/ and https://www.dst.dk/en/TilSalg/Forskningsservice/Dataadgang.
The full-text version of this article contains a data supplement.