TO THE EDITOR:
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) typically utilizes fresh donor bone marrow (BM) or peripheral blood stem cells (PBSCs) because of concerns that cryopreservation may negatively impact patient outcomes.1 The COVID-19 pandemic spurred renewed interest in the impact of cryopreservation after the National Marrow Donor Program (NMDP) mandated cryopreservation of unrelated donor (URD) PBSCs between March and August 2020.2
Although the cryopreservation mandate was lifted after August 2020, heterogeneity in cryopreservation practices among transplant centers remains. Moreover, data on long-term outcomes of using such products are scarce. We previously reported lack of difference in 6-month overall survival (OS), progression free survival (PFS), relapse, and nonrelapse mortality (NRM) for adult recipients of cryopreserved PBSCs compared with those of fresh URD PBSCs, noting lower T-cell chimerism at days 30 and 100.3,4 We and others reported delayed neutrophil and platelet reconstitution with cryopreservation,3,5 whereas other investigators found no difference.6,7 Although some studies have reported increased moderate/severe chronic graft versus host disease (cGVHD) after allo-HSCT with cryopreserved PBSCs,5,6 others have described equivalent or slightly reduced cGVHD incidence,7,8 although differences in GVHD prophylaxis regimens and small sample sizes may account for this variability.
As lower white blood cell count and T-cell chimerism at 30- and 100-days following allo-HSCT are associated with relapse,9,10 long-term implications of impaired immune reconstitution after stem-cell product cryopreservation warranted further investigation. We have expanded our cohort to 387 adult patients (136 cryopreserved, 251 fresh) treated with URD PBSC and assessed 2-year clinical outcomes including OS, PFS, relapse, NRM, and acute and chronic GVHD (aGVHD, cGVHD).
This study was approved by the Institutional Review Board of Dana-Farber/Harvard Cancer Center (DF/HCC) and was conducted in accordance with the Declaration of Helsinki. We analyzed all adult patients who underwent the first allo-HSCT with URD PBSCs between January 1, 2019 and July 31, 2021 (136 cryopreserved PBSC, 251 fresh). Of the 387 patients, 304 were included in our earlier reports limited to short-term clinical outcomes.3,4 Indication for cryopreservation was COVID-19 precautions (clinician preference and/or NMDP mandate) for 128 of 136 patients. For the remaining 8 patients receiving cryopreserved product before March 2020, the indication was donor scheduling.
Baseline and pretransplant operational characteristics were reported descriptively and compared using Fisher’s exact test, χ2 test, or Wilcoxon rank-sum test, as appropriate. Clinical outcomes of interest included OS, PFS, NRM, relapse, aGVHD, and cGVHD. OS was defined as time from stem cell infusion to death from any cause. PFS was defined as time from stem cell infusion to disease relapse, progression, or death from any cause, whichever occurred first. Patients who were alive without disease relapse or progression were censored at the time last seen alive and relapse or progression-free. The Kaplan-Meier method was used to estimate PFS and OS. Cumulative incidence of NRM, relapse, and GVHD were estimated in the context of a competing risks framework. NRM and relapse were treated as competing events to each other and death or relapse without developing GVHD served as a competing event for GVHD. All calculations were performed using SAS 9.3 (SAS Institute, Inc, Cary, NC) and R version 3.3.2.
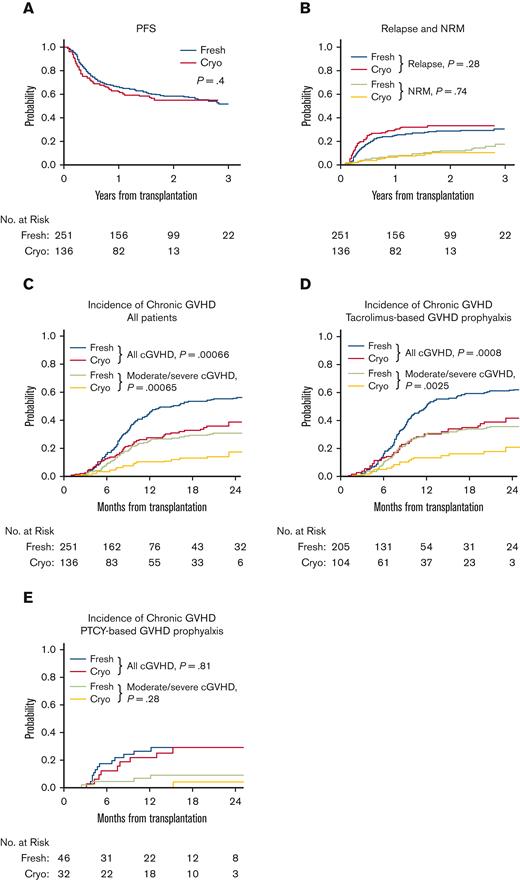
Characteristics of patients, donors, and transplant regimens were similar between cohorts (Table 1). Median follow-up time among survivors was 23.1 months (range, 12-40.9 months). Two-year OS (60% versus 65%, P = .64) and PFS (55% versus 58%, P = .4) were not different in patients receiving cryopreserved PBSCs compared with those receiving fresh PBSCs (Table 1, Figure 1A). Relapse (34% versus 29%, P = .28) and NRM (11% versus 12%, P = .74) at 2 years and graft failure (5.1% versus 2%, P = .18) were comparable between cryopreserved and fresh cohorts (Figure 1B). Eight patients died of COVID-19 (3 cryopreserved, 5 fresh). At 1 year, T-cell chimerism was not different between groups (Table 2). Incidence of grade II-IV and grade II-IV aGVHD at 6 months did not differ between cryopreserved and fresh cohorts (grade II-IV: 25% versus 20%, P = .36; grade III-IV: 12% versus 8%, P = .35; Table 2). Strikingly, 2-year incidence of any grade cGVHD and moderate/severe cGVHD was lower in patients receiving cryopreserved PBSCs than in those receiving fresh PBSCs (any grade: 39% versus 57%, P = .00066; moderate/severe: 18% versus 31%, P =.00065; Figure 1C). This difference was restricted to patients receiving a tacrolimus+methotrexate and/or sirolimus GVHD prophylaxis regimen (any grade: 42% versus 61%, P = .0008; moderate/severe: 21% versus 36%, P = .0025; Figure 1D) but not those receiving posttransplant cyclophosphamide for GVHD prophyaxis (any grade: 29% versus 29%, P = .81; moderate/severe: 4.2% versus 9.3%, P = .28; Figure 1E). Multivariable analysis showed an increased subdistribution hazard ratio (sHR) for cGVHD for fresh versus cryopreserved PBSCs, donor age ≥40, and patient/donor CMV positivity (Table 3). This difference in cGVHD incidence was observed both during and after the NMDP-mandated cryopreservation period. The median duration of cryopreservation was 262.7 hours (range 48.4 to 2855.7). We observed no difference in cGVHD based on duration of cryopreservation (data not shown).
Clinical outcomes of patients receiving cryopreserved versus fresh PBSCs. PFS (A), NRM (B), and relapse (B) were similar between patients receiving fresh (blue or green) versus cryopreserved (red or orange) PBSCs. Incidence of all grade cGVHD and moderate/severe cGVHD was lower in patients receiving cryopreserved PBSCs (red or orange) compared with those receiving fresh PBSCs (blue or green) (C). This difference was only observed in patients treated with tacrolimus+methotrexate +/− sirolimus for GVHD prophylaxis (D) but not in patients given PTCY for GVHD prophylaxis (E).
Clinical outcomes of patients receiving cryopreserved versus fresh PBSCs. PFS (A), NRM (B), and relapse (B) were similar between patients receiving fresh (blue or green) versus cryopreserved (red or orange) PBSCs. Incidence of all grade cGVHD and moderate/severe cGVHD was lower in patients receiving cryopreserved PBSCs (red or orange) compared with those receiving fresh PBSCs (blue or green) (C). This difference was only observed in patients treated with tacrolimus+methotrexate +/− sirolimus for GVHD prophylaxis (D) but not in patients given PTCY for GVHD prophylaxis (E).
PBSC cryopreservation is an attractive option allowing greater flexibility in timing and coordination of donor collection and allo-HSCT not only during the COVID-19 pandemic but perhaps beyond. We now report that cryopreservation is associated with lower cumulative incidence of any grade cGVHD and moderate/severe cGVHD without compromising relapse in patients who receive tacrolimus+methotrexate and/or sirolimus GVHD prophylaxis but not in those who receive PTCY. Initially pioneered to improve engraftment and reduce GVHD in haploidentical allo-HSCT,11 PTCY-based GVHD prophylaxis is gaining traction for reducing incidence of cGVHD in matched related or unrelated allo-HSCT.12-14 PTCY is hypothesized to prevent GVHD by limiting alloreactivity in donor T cells, favoring more rapid recovery of regulatory T cells and delaying CD4+ conventional T-cell reconstitution, thereby inducing tolerance and abrogating inflammation.15-17 Our previous analysis of T-cell reconstitution and immunophenotyping between recipients of fresh and cryopreserved PBSCs demonstrated lower absolute numbers of conventional naïve and effector memory (EM) T cells at 30 and 100 days following allo-HSCT and a trend toward decreased numbers of conventional central memory, effector memory reexpressing RA (EMRA) T cells, and Tregs after cryopreserved PBSCs.3 Although the ratio of regulatory to conventional T cells was preserved, there was a trend toward a higher ratio at day 100 (regulatory to conventional T-cell ratio: 0.12 for cryopreserved versus 0.09 for fresh, P = .06). Given that cryopreservation appears to offer similar protection from GVHD compared with that offered by PTCY, a question for future investigation is whether cryopreservation selectively impairs similar T-cell functional subsets as PTCY.
We show that allo-HSCT with cryopreserved URD PBSCs results in similar OS, PFS, relapse rates, and NRM at 2 years posttransplantation. Despite early impairment of T-cell chimerism, previously linked to higher relapse,10 we do not observe differences in longer term relapse rates. We hypothesize that reduction in T-cell reconstitution caused by cryopreservation is fundamentally different from impaired T-cell reconstitution predisposing to relapse. Further analysis is needed of specific T-cell and other lymphocyte phenotypes in cryopreserved and fresh PBSC recipients who either relapse or have sustained remission to fully dissect these differences. Such an analysis may elucidate functional subsets of T cells required for maintenance of a graft-versus-leukemia effect and incidence of cGVHD after allo-HSCT. Multicenter or registry analyses are needed to confirm the clinical observations reported here.
Acknowledgments: The authors acknowledge the National Donor Marrow Program (NMDP) for their efforts to maintain access to donors throughout the ongoing pandemic and the DF/HCC clinical oncology support staff for continued care of these patients. The authors also thank the Connell and O'Reilly Families Cell Manipulation Core Facility (CMCF) at DFCI for continuous efforts in processing stem cell products and for providing data for this manuscript. This work was supported by the National Cancer Institute, National Institutes of Health (P01CA229092).
Contribution: K.M., R.J.S., and S.N. conceived the project and designed the study; H.T.K. designed, performed, and interpreted the statistical analysis; K.M., H.G., D.L., and H.T.K. participated in identification, collection, and interpretation of patient information; K.M., H.T.K, H.G., D.L., C.C., J.H.A., J.K., J.R., R.M.S., R.R., C.J.W., R.J.S, V.T.H., M.G., and S.N. participated in manuscript writing and review; and all authors provided final approval of the manuscript.
Conflict-of-interest disclosure: C.J.W holds equity in BioNTech, and receives research funding from Pharmacyclics. J.R. received research funding from Amgen, Equillium, Kite Pharma, and Novartis and served on advisory boards for Akron, AVROBIO, Clade, Garuda, Immunitas, LifeVault, Rheos, Talaris, and TScan. J.K. reports research support from Amgen, Equillium, BMS, Miltenyi Biotec, Regeneron, and Clinigen and consulting income from Amgen, Equillium, and Moderna Therapeutics and is a scientific advisory board member for Cugene and Therakos. S.N. reports ad hoc advisory boards for Kite/Gilead, GlaxoSmith Kline, Iovance, A2 Bio, and Sobi. R.J.S. serves on the board of directors for Be The Match/National Marrow Donor Program; provided consulting for Vor Biopharma, Neovii, CSL Behring, Bluesphere Bio, Cugene, Jasper, Smart Immune; and is on the Data Safety Monitoring board for Juno Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Sarah Nikiforow, Dana-Farber Cancer Institute; 450 Brookline Ave, Smith 1101, Boston, MA 02215; e-mail: sarah_nikiforow@dfci.harvard.edu.