TO THE EDITOR:
Human liver endothelial cells (HLEC) line the hepatic sinusoids and are reported to have specialized functions, which underscores their importance in human physiology. Specifically, HLEC regulate hemostasis by producing factor VIII (FVIII).1 HLEC participate in the immune response,2,3 and eliminate waste products from the blood owing to their endocytic and lysosomal capacity.4,5 Recent literature has shown that HLEC are a heterogeneous cell population. Indeed, single cell transcriptomics and immunostaining experiments have categorized HLEC into 2 major groups according to their location within the liver lobules (zone 1 or periportal HLEC; and zones 2 or 3 or central venous [CV] HLEC).6,7 In this study, we integrate public bulk and single cell transcriptomic data and perform in vitro experiments to characterize the functions of HLEC subtypes, compare them to large vessel endothelial cells and identify putative transcription factors (TFs) that drive their distinct characteristics.
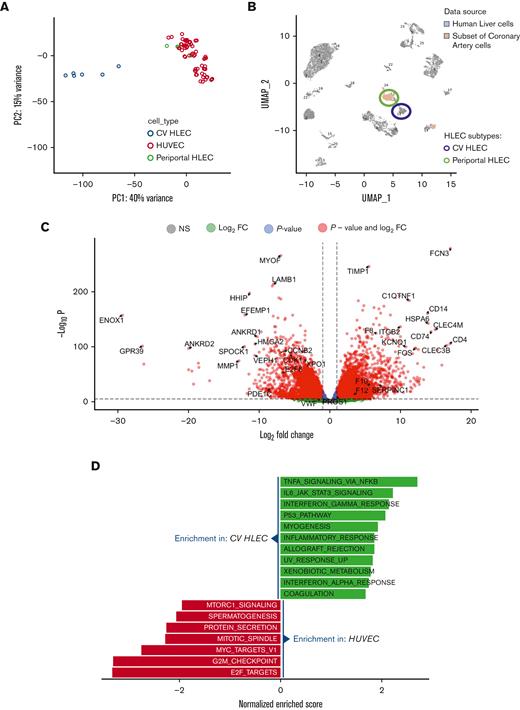
The primary goal of our study was to understand what distinguishes specialized HLEC from large vessel endothelium. We uniformly processed all bulk RNA sequencing (RNA-seq) data from HLEC that are available in Gene Expression Omnibus and compared their characteristics to those of the most common vascular endothelial cell subtype, human umbilical vein endothelial cells (HUVEC). We analyzed data from 10 HLEC samples and 108 HUVEC samples from 37 independent experiments. Principal component analysis of those samples revealed that only 6 of 10 HLEC samples had robust transcriptional differences compared with HUVEC (Figure 1A). Concordant with prior literature,7 these data suggest that there are at least 2 distinct subpopulations of endothelial cells in human liver, 1 endothelial cell type with gene expression similar to HUVEC and another with gene expression different from HUVEC.
Transcriptional differences between HLEC and vascular endothelial cells. (A) PCA of bulk RNA-seq results. CV HLEC and periportal HLEC were defined according to CIBERSORTx results. (B) Uniform Manifold Approximation and Projection (UMAP) showing the projection of single cell RNA-seq of arterial endothelial cells onto human liver single cell RNA-seq data. Note that most of the arterial endothelium projects to periportal HLECs and none projects to the CV HLEC cluster. (C) Volcano plot presenting the transcriptional differences between CV HLEC and HUVEC (CV HLEC upregulated genes on the right). (D) Gene set enrichment analysis revealing the HALLMARK enriched pathways in CV HLEC and HUVEC at FDR <0.05.
Transcriptional differences between HLEC and vascular endothelial cells. (A) PCA of bulk RNA-seq results. CV HLEC and periportal HLEC were defined according to CIBERSORTx results. (B) Uniform Manifold Approximation and Projection (UMAP) showing the projection of single cell RNA-seq of arterial endothelial cells onto human liver single cell RNA-seq data. Note that most of the arterial endothelium projects to periportal HLECs and none projects to the CV HLEC cluster. (C) Volcano plot presenting the transcriptional differences between CV HLEC and HUVEC (CV HLEC upregulated genes on the right). (D) Gene set enrichment analysis revealing the HALLMARK enriched pathways in CV HLEC and HUVEC at FDR <0.05.
Given the heterogeneity of HLEC subtypes, we hypothesized that cell subtype composition of bulk HLEC may be the underlying reason for the observed differences of bulk HLEC transcriptomes. To test that hypothesis, we used CIBERSORTx8 to perform cell type deconvolution of bulk HLEC RNA-seq using a human liver single cell RNA-seq dataset.7 CIBERSORTx showed that the HLEC samples which were identified as distinct from the vascular endothelium by principal component analysis (PCA) correspond largely to CV HLECs, whereas the remaining HLEC and HUVEC samples are most similar to periportal HLEC (supplemental Figure 1A).
The similarity of periportal HLEC to large vessel endothelial cells in our analysis was an unexpected finding, so we attempted to validate this finding using orthogonal single cell data. We mapped endothelial cells identified in human coronary artery single cell RNA-seq9 onto cell clusters of human liver single cell RNA-seq.7 We found that the most of the vascular endothelial cells map onto the periportal HLEC cluster (Figure 1B), suggesting that the transcriptional program of periportal HLEC has a high degree of similarity to vascular endothelial cells. In contrast, none of the arterial vascular endothelial cells mapped onto the CV HLEC cluster, indicating the distinct character of CV HLEC. These data validate the finding that periportal liver endothelial cells are largely similar to large vessel endothelial cells and emphasize the unique transcriptional signature of CV HLEC. Additional comparisons of HLEC subtype proportions in healthy and diseased livers demonstrate that CV HLEC are decreased in livers of patients with hepatocellular carcinoma, whereas both CV and periportal HLEC are decreased in patients with cirrhosis (supplemental Figure 1B).
To assess the unique characteristics of CV HLEC, we performed differential expression analysis between the HLEC identified as CV HLEC and HUVEC. Our analysis identified 5613 genes under-expressed and 4470 genes overexpressed in HLEC compared with HUVEC at a false discovery rate (FDR) <0.05 (Figure 1C; supplemental Table 1). Gene set enrichment analysis10,11 revealed an enrichment of inflammatory and coagulation pathways in CV HLEC whereas proliferation and mitosis pathways were increased in HUVEC (Figure 1D; supplemental Table 2). A similar analysis comparing bulk HLEC identified as periportal HLEC and HUVEC did not reveal an enrichment for coagulation or inflammatory pathways (supplemental Figure 1C; supplemental Tables 3-4) whereas a comparison of CV HLEC and periportal HLEC confirmed enrichment for coagulation pathways in CV HLEC (supplemental Figure 1D,E; supplemental Tables 5-6). Notably, consistent with prior literature,12 we found that CV HLEC can be identified based on Fc gamma receptor IIb (FCGR2B) expression which appears to be uniquely present in this subset of HLEC (supplemental Figure 1F). We used FCGR2B as a marker for cell sorting of CV HLEC in the studies described below.
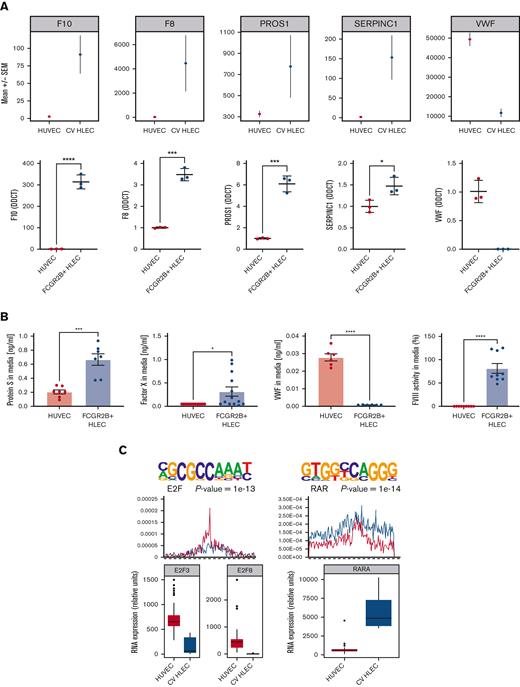
We then proceeded to evaluate CV HLEC expression of coagulation proteins. Our RNA-seq results showed that CV HLEC express higher levels of 4 key coagulation genes (F8, F10, PROS1, and SERPINC1) compared with HUVEC and lower levels of von Willebrand factor (VWF) (Figure 2A). We purified CV HLEC by sorting commercially obtained HLEC based on surface FCGR2B expression (supplemental Figure 1G). We validated higher levels of those coagulation genes (F8, F10, PROS1, and SERPINC1) and lower expression of VWF in FCGR2B+ HLEC than in HUVEC (Figure 2A). Similarly, we confirmed higher levels of coagulation genes (F8 and F10) in FCGR2B+ than in FCGR2B- HLEC (supplemental Figure 2A). We then measured the amount of those factors at the protein level secreted into the media by enzyme-linked immunosorbent assay. Compared with HUVEC, FCGR2B+ HLEC showed a significant increase in release of Protein S and FX, whereas VWF levels were not detected in the supernatants of FCGR2B+ HLEC. FVIII activity levels were also significantly increased in the supernatants of FCGR2B+ HLEC compared with that of HUVEC (Figure 2B), whereas both FVIII activity and FX release were significantly increased in FCGR2B+ HLEC compared with FCGR2B- HLEC (supplemental Figure 2B). To further understand the difference between VWF and FVIII expression in CV HLEC we compared bulk RNA-seq data from multiple human endothelial cell lines from the Gene Expression Omnibus database (supplemental Table 7) finding that VWF is expressed in multiple endothelial cell types and its expression is generally higher in large vessels than in the microvasculature, whereas FVIII expression is largely constrained to CV HLEC (supplemental Figure 2C). Taken together, our results suggest that CV HLEC may play an important role in the regulation of coagulation.
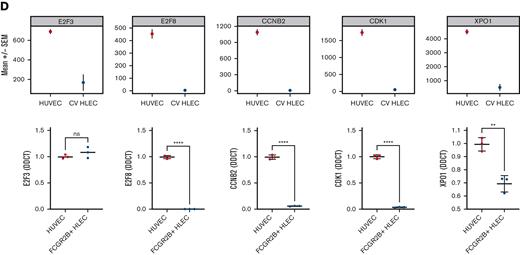
Expression of coagulation factors in HLEC vs HUVEC and prioritized transcription factor drivers. (A) Bulk RNA-seq (upper panel) and reverse transcriptase quantitative polymerase chain reaction based (lower panel) expression of coagulation genes (F8, F10, PROS1, SERPINC1, VWF) in CV HLEC and HUVEC (results presented as mean +/− standard error of the mean). (B) Enzyme-linked immunosorbent assay of 24 hour supernatants of HUVEC and FCGR2B+ HLEC for PROS1, FX, VWF, and activity assay for FVIII. (C) Prioritized denovo motifs enriched in genes downregulated (left) or upregulated (right) in CV HLEC compared with HUVEC (top panel). Corresponding enrichment of known E2F and RXR:RAR motifs in CV HLEC and HUVEC (middle panel), box plot with the expression of E2F3, E2F8 and RARA in CV HLEC and HUVEC in bulk RNA-seq (lower panel). (D) Bulk RNA-seq (upper panel) and reverse transcriptase quantitative polymerase chain reaction based (lower panel) expression of E2F pathway genes (E2F3, E2F8, CCNB2, CDK1, XPO1) in CV HLEC and HUVEC (results presented as mean +/− standard error of the mean).
Expression of coagulation factors in HLEC vs HUVEC and prioritized transcription factor drivers. (A) Bulk RNA-seq (upper panel) and reverse transcriptase quantitative polymerase chain reaction based (lower panel) expression of coagulation genes (F8, F10, PROS1, SERPINC1, VWF) in CV HLEC and HUVEC (results presented as mean +/− standard error of the mean). (B) Enzyme-linked immunosorbent assay of 24 hour supernatants of HUVEC and FCGR2B+ HLEC for PROS1, FX, VWF, and activity assay for FVIII. (C) Prioritized denovo motifs enriched in genes downregulated (left) or upregulated (right) in CV HLEC compared with HUVEC (top panel). Corresponding enrichment of known E2F and RXR:RAR motifs in CV HLEC and HUVEC (middle panel), box plot with the expression of E2F3, E2F8 and RARA in CV HLEC and HUVEC in bulk RNA-seq (lower panel). (D) Bulk RNA-seq (upper panel) and reverse transcriptase quantitative polymerase chain reaction based (lower panel) expression of E2F pathway genes (E2F3, E2F8, CCNB2, CDK1, XPO1) in CV HLEC and HUVEC (results presented as mean +/− standard error of the mean).
To further understand the drivers of the distinct transcriptome of CV HLEC, we performed a TF prioritization approach using HOMER motif enrichment13 (Figure 2C). We prioritized TFs whose motifs are enriched in genes upregulated in CV HLEC or HUVEC and whose expression level correlates with motif enrichment. This approach assumes that for activating TFs, higher gene expression of the TF should correlate with upregulation of their target genes, with the opposite being true for repressor TFs. This analysis showed that retinoid acid receptor (RAR) TFs were upregulated in CV HLEC compared with HUVEC and their corresponding motifs were enriched in CV HLEC upregulated genes (Figure 2C). Similarly, the inhibitory TF ZNF263 was upregulated in CV HLEC and its corresponding motifs were enriched in CV HLEC downregulated genes (supplemental Figure 2D). In contrast, E2F family TFs (E2F8 and E2F3) were highly upregulated in HUVEC compared with that in CV HLEC with strong enrichment of the E2F motif (Figure 2C). We validated the E2F pathway using reverse transcriptase quantitative polymerase chain reaction and upregulation was found in FCGR2B+ HLEC compared with HUVEC by testing known genes in the pathway14,15 (E2F3, E2F8, CCNB2, CDK1, and XPO1), which largely confirmed our computational results and nominated E2F8 as the most promising TF driving the differences (Figure 2D). We also showed that E2F8 and its downstream genes CCNB2, CDK1, and XPO1 are increased in FCGR2B- compared with FCGR2B+ HLEC providing further validation for the transcriptional similarities between HUVEC and periportal HLEC (supplemental Figure 2E). We subsequently silenced E2F8 in HUVEC and found increased F8 gene expression, and significantly impaired VWF release in response to histamine (supplemental Figure 2F). In aggregate, these analyses suggest a potential role for E2F8 as a determinant of the differences between HUVEC and CV HLEC in hemostasis.
The major finding of our study was that CV HLEC are a distinct subpopulation of liver endothelial cells with a unique transcriptional signature. HLEC zonation was previously demonstrated by others.16,17 Our findings provide experimental in vitro support for the distinct characteristics of HLECs from different zones and provide a framework for in vitro exploration of HLEC subtypes. We show that CV HLECs are transcriptionally divergent from the large vessel endothelium and produce and release coagulation factors such as FX and FVIII. In contrast, periportal HLEC are transcriptionally similar to large vessel endothelium, pointing to potential distinct embryologic origins of the 2 subtypes.
In addition, we identifed downregulation of E2F8 as a potential driver of the unique features of the CV HLEC transcriptome and additionally nominate RAR family TFs and ZNF263 as TFs that should be further evaluated in future studies. Interestingly, concordant with our data, previous studies on the development of liver endothelial cells in mice have identified RARB as a candidate TF regulating liver endothelial specification,18 whereas work by others have nominated additional TFs like GATA4, C-MAF, and MEIS2 as potential regulators of certain HLEC features.19 Future work in endothelial cell differentiation in vitro and in vivo will be needed to further explore those TFs and identify the drivers of unique HLEC features and subtypes.
Our data may have important implications in the future management of human coagulopathies. First, the role of CV HLEC as producers of multiple hemostasis genes and their preferential damage in diseases like cirrhosis and hepatocellular carcinoma, suggest the need for additional research into the consequences of selective damage of CV HLEC in vivo and subsequently the potential for regenerative therapies targeted at CV HLEC in disease. Second, by experimentally confirming the role of CV HLEC as central producers of FVIII, our data provide a potential new target cell type for gene therapies against hemophilia A which have so far been focusing on hepatocytes with mixed results.20,21 Our findings point to an initial set of genes that could be used for targeted therapies focusing on CV HLEC in the liver, including FCGR2B, CLEC1B, and STAB2. However, further in vivo work confirming our ability to target CV HLEC and the consequences of that targeting will be necessary before clinical implementation. Overall, our findings emphasize the need for additional research evaluating the unique roles and characteristics of distinct liver endothelial cell subtypes in human physiology and disease.
Acknowledgments: This project was supported by American Heart Association grant #940488 (M.A.) and National Institutes of Health grants R01HL134894 and R33HL141791 (C.J.L.). The authors thank the JHSPH cell sorting laboratory personnel (Johns Hopkins University), especially Hao Zhang for their contribution to this work.
Contribution: P.T.-S. and P.R. performed the research, analyzed data, and wrote the manuscript; M.V., W.O.O., and N.A. reviewed the manuscript and helped to perform the research; C.J.L. provided general oversight and supervision, helped with study design, and reviewed the manuscript; M.A. provided general oversight and supervision, conceptualized and designed the research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marios Arvanitis, Division of Cardiology, Johns Hopkins School of Medicine, 720 Rutland Ave, Ross 844, Baltimore, MD 21205; e-mail: marvani1@jhmi.edu.
References
Author notes
Data are available on request from the corresponding author, Marios Arvanitis (marvani1@jhmi.edu).
The full-text version of this article contains a data supplement.
P.T.-S., P.R., C.J.L., and M.A. contributed equally to this work.