TO THE EDITOR:
Ten to 15% of patients with diffuse large B-cell lymphoma (DLBCL) have primary refractory disease and 20% to 30% relapse.1 For patients who are eligible for stem cell transplantation (SCT), second-line chemotherapy and autologous SCT is curative in around 20%, whereas third-line anti-CD19 chimeric antigen receptor (CAR) T-cell therapy results in durable remissions in up to 40% of treated patients.2-4
For patients who are not eligible for SCT or CAR T-cell therapy and those with progressive disease (PD) after CAR T-cell therapy, outcomes remain poor and new approaches are required.1 When CAR T-cell therapy is planned, up to 20% of patients do not go on to receive the product, often because of PD, and novel bridging strategies are needed for this largely chemotherapy-refractory group.5-8
In the randomized phase 2 G029365 trial, polatuzumab vedotin (an anti-CD79b monoclonal antibody conjugated to the cytotoxin monomethyl auristatin E) with bendamustine and rituximab (Pola-BR) was compared with bendamustine-rituximab for treatment of relapsed/refractory (R/R) DLBCL in patients ineligible for SCT. For Pola-BR, the objective response rate (ORR) was 62.5% (complete response [CR] rate was 50%). Median progression-free survival (PFS) a at 9.2 months and overall survival (OS) at 12.4 months were both superior for Pola-BR.9 A single-arm expansion cohort identified primary refractory disease, >1 previous treatments, and refractoriness to the last treatment as predictors of inferior PFS and OS.10
Before regulatory approval in the United Kingdom, Pola-BR was available via the Early Access to Medicines Scheme (EAMS) between June 2019 and January 2020 in line with the intended label.11 Subsequently, interim funding (from March to August 2020) was provided via the Cancer Drugs Fund (CDF) for Pola-BR because of potential delays in CAR T-cell therapy delivery during the Covid-19 pandemic. We analyzed outcomes of patients treated on these schemes.
Anonymized data were collected retrospectively from 28 United Kingdom hospitals for consecutive patients treated with Pola-BR via EAMS or CDF interim funding. All patients who started Pola-BR treatment via either scheme were eligible (for EAMS: patients with R/R DLBCL after ≥1 previous treatments who were ineligible for SCT; for CDF: patients with R/R DLBCL after ≥2 previous treatments and who were approved to receive CAR T-cell therapy). Polatuzumab was given on day 1 or 2 of a 28-day cycle at a dose of 1.8 mg/kg for a maximum of 6 cycles. Dose reduction or treatment delay because of adverse events was permitted according to physician discretion. Response assessment was performed according to local policy.
The collection and analysis of the data were part of routine National Health Service (NHS) evaluation and did not require ethical review. Full methods, statistical analysis, and treatment details are listed in the supplemental Data.
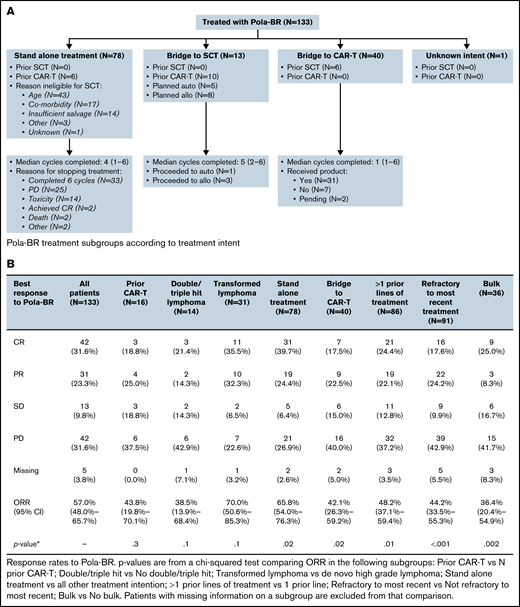
Data were collected from 133 patients (EAMS, n = 106; CDF, n = 27) treated from June 2019 to October 2020. Treatment intent was bridging to CAR T-cell therapy for 30.1% (n = 40), re-induction therapy with planned SCT consolidation for 9.8% (n = 13), and stand-alone treatment (no planned CAR T-cell therapy or SCT) for 58.6% (n = 78). Table 1 summarizes patient characteristics. Figure 1A shows subgroups according to treatment intent.
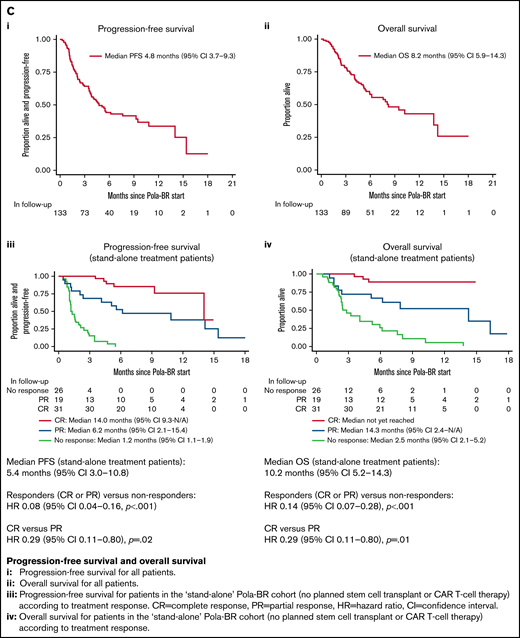
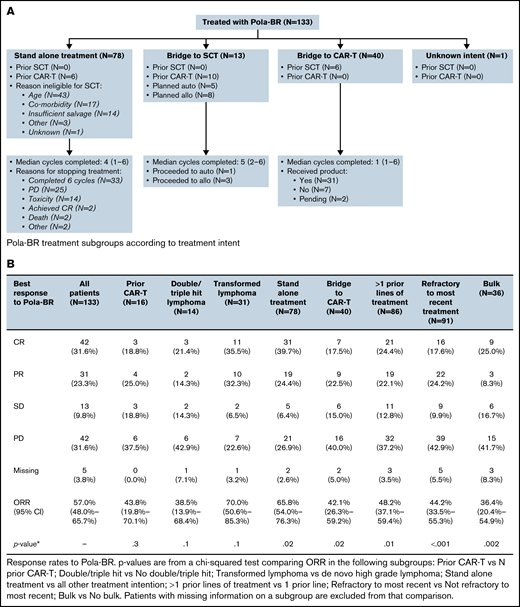
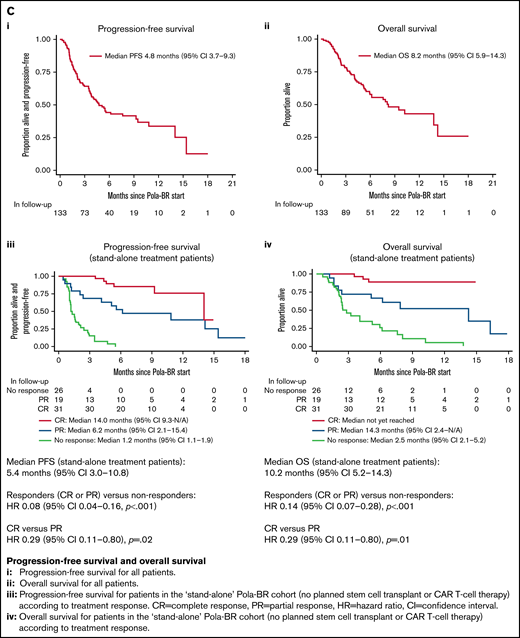
Treatment subgroups according to treatment intent, treatment response rates, PFS and OS. (A) Pola-BR treatment subgroups according to treatment intent. (B) Response rates to Pola-BR. (C) PFS and OS. (i) PFS and (ii) OS for all patients. (iii) PFS for patients in the stand-alone Pola-BR cohort (no planned SCT or CAR T-cell therapy) according to treatment response. CI, confidence interval; HR, hazard ratio; PR, partial response. (iv) OS for patients in the stand-alone Pola-BR cohort (no planned SCT or CAR T-cell therapy) according to treatment response.
Treatment subgroups according to treatment intent, treatment response rates, PFS and OS. (A) Pola-BR treatment subgroups according to treatment intent. (B) Response rates to Pola-BR. (C) PFS and OS. (i) PFS and (ii) OS for all patients. (iii) PFS for patients in the stand-alone Pola-BR cohort (no planned SCT or CAR T-cell therapy) according to treatment response. CI, confidence interval; HR, hazard ratio; PR, partial response. (iv) OS for patients in the stand-alone Pola-BR cohort (no planned SCT or CAR T-cell therapy) according to treatment response.
A median of 4 cycles (range, 1-6 cycles) were given (median, 1 for CAR T-cell therapy bridging vs 4 for stand-alone treatment, and 5 when SCT consolidation was planned). Pola-BR was initiated with full-dose bendamustine for 91 patients (68.4%), reduced-dose bendamustine for 24 patients (18.0%), bendamustine was omitted for 5 patients (3.8%), and data were missing for 13 patients (9.8%). The investigator-assessed best ORR was 57.0% (CR, 31.6%). Response rates for predefined subgroups are provided in Figure 1B. Median follow-up duration was 7.7 months, median PFS was 4.8 months (95% confidence interval [CI], 3.7-9.3 months), and median OS was 8.2 months (95% CI, 5.9-14.3 months) (Figure 1C).
For stand-alone Pola-BR (no planned CAR T-cell therapy or SCT [n = 78]), a majority of patients were ineligible for SCT because of age (55.1%) or comorbidities (21.8%), but for 17.9% of patients, this was a result of insufficient response to previous therapy. Primary reasons for treatment discontinuation were completion of 6 cycles (n = 33; 42.3%), PD (n = 25; 32.1%), treatment-related toxicity (n = 14; 17.9%), patient death (n = 2; 2.6%), achieving CR (n = 2; 2.6%), and other (n = 2; 2.6%).
In the stand-alone group, 26 patients (33.3%) experienced treatment delay because of adverse events, most commonly infection (n = 14; 17.9%) and hematologic toxicity (n = 11; 14.1%), as well as nausea (n = 1), diarrhea (n = 1), fatigue (n = 4), and peripheral neuropathy (PN; n = 1). Bendamustine dose was reduced for 33 patients (42.3%) and omitted for 4 patients (5.1%) in at least 1 cycle. Reported reasons for bendamustine dose reduction or omission were hematologic toxicity (n = 7), patient age (n = 7), infection (n = 6), frailty (n = 4), diarrhea (n = 3), increased bilirubin (n = 2), infusion-related reaction (n = 1), comorbidities (n = 1), and unknown (n = 6). One patient (1.3%) discontinued treatment because of PN. When data were available, 16 (27.1%) of 59 patients required admission to the hospital because of toxicity related to Pola-BR during treatment.
The ORR for the stand-alone cohort was 65.8% (CR, 39.7%), the median follow-up duration was 8.2 months, median PFS was 5.4 months (95% CI, 3.0-10.8 months), and median OS was 10.2 months (95% CI, 5.2-14.3 months). The 12-month PFS rate was 37% (95% CI, 24%-50%). For patients achieving CR, median PFS was 14.0 months and median OS was not reached (Figure 1C). For this stand-alone group, significant factors by univariable analysis for shortened PFS were bulky disease (>7.5 cm) (hazard ratio [HR], 2.32; 95% CI, 1.23-4.38; P = .009), >1 previous treatment (HR, 2.17; 95% CI, 1.19-3.95; P = .01), and refractoriness to the last treatment (HR, 3.48; 95% CI, 1.79-6.76; P < .001). Significance was maintained in a multivariable model using these 3 variables.
Pola-BR was planned as bridging to CAR T-cell therapy for 40 patients: 31 (77.5%) of 40 received cell infusion (18, axicabtagene ciloleucel; 12, tisagenlecleucel; and 1, clinical trial product), 5 died as a result of PD, 1 died as a result of infection during bridging, CAR T-cell therapy infusion was pending for 2, and data were missing for 1. Leukapheresis occurred before bridging for 36 patients (90.0%) and after at least 1 cycle of Pola-BR for 3 (10.0%) (of these, 2 patients received bendamustine and both underwent successful leukapheresis). The best ORR to Pola-BR bridging was 42.1% (CR, 17.5%; partial response [PR], 22.5%; stable disease [SD], 15.0%; PD, 40.0%; missing, 5.0%). Sixteen patients received Pola-BR having progressed after CAR T-cell therapy. The ORR was 43.8% and the CR rate was 18.8%; 3 of 16 patients subsequently proceeded to allogeneic SCT. In total, 4 patients underwent SCT after treatment with Pola-BR (3 allogeneic, 1 autologous). Sixty patients died during follow-up, including 48 as a result of PD and 6 as a result of infection during Pola-BR treatment.
These outcome data for 133 consecutive patients treated with Pola-BR add substantially to evidence from the registration trial and other studies.9,10,12-15 Within its limitations (investigator-reported outcomes and limited toxicity data), this retrospective study supports Pola-BR as a treatment for patients with R/R DLBCL who are ineligible for SCT and provides preliminary evidence of efficacy for CAR T-cell therapy bridging and after CAR T-cell therapy failure.
For stand-alone Pola-BR treatment without planned consolidation, the ORR (57.1%; 95% CI, 54.0%-76.3%) is comparable to that reported in G029365 (62.5%), although fewer patients attained CR (39.7% vs 50%). Median PFS (5.4 months, 95% CI, 3.0-10.8 months) and median OS (10.2 months; 95% CI, 5.2-14.3 months) are shorter than in the trial (median PFS: 9.2 months; 95% CI, 6.2-13.9 months; median OS: 12.4 months; 95% CI, 9 months to not estimable).9 The short median PFS in this group may reflect the frequency of high-risk features; more patients were ineligible for SCT because of age, comorbidities, or performance status (PS) than in the trial (78.2% vs 35.0%), and many had bulk disease (28.2%), high International Prognostic Index (IPI) score (71.8%) or PS ≥2 (39.7%). Just 17.9% of patients in this group were ineligible for SCT because of insufficient response to previous treatment compared with 30.0% of patients in the trial. Unsurprisingly, PFS was shorter after >1 previous treatment and for patients refractory to the preceding treatment. Bulky disease was also associated with inferior PFS, a finding not previously reported from G029365. For those achieving CR, median PFS was 14.0 months (median OS was not reached), but further follow-up is required to have confidence in the durability of CR. The limited toxicity data available for this stand-alone group are overall in keeping with the known safety profile of Pola-BR; however, it is notable that as a result of treatment-associated toxicity, 17.9% of patients in the stand-alone treatment group stopped Pola-BR before completing 6 cycles and 27.1% required hospital admission. Although response rates for the 16 patients who received Pola-BR for PD after CAR T-cell therapy were lower than for the whole cohort (ORR, 43.8%; CR, 18.8%), few other treatments have been tested in this setting, and it is notable that 3 of 16 patients were successfully bridged to allogeneic SCT.
A median of 1 cycle was given as bridging to CAR T-cell therapy. The ORR was 42.1% and the CR rate was 18.4%, similar to rates for the post-CAR T-cell therapy group. A majority (31 [77.5%] of 40) proceeded to cell infusion; thus, Pola-BR seems to be a feasible bridging strategy. Further studies are required to define who is most likely to benefit from this treatment and to assess other approaches for this chemotherapy-refractory group.16 -18
This is the largest data set of patients treated with Pola-BR to date. Other series report broadly similar outcomes, but this study is unique in its sample size and inclusion of patients at different stages in the DLBCL treatment pathway.12-15 These outcomes support Pola-BR as a treatment for patients who are ineligible for SCT, help to delineate which groups stand to benefit most, and show efficacy in transformed low-grade lymphoma and double-hit lymphoma which were not represented in the G029365 trial. Furthermore, these data offer new insights into the role of Pola-BR as CAR T-cell therapy bridging and as treatment after CAR T-cell therapy failure. PFS seems shorter in this study than in the G029365 trial, which possibly reflects patient characteristics, including an older median age in this study (72 vs 67 years), a higher proportion of patients with PS ≥2 (30.1% vs 15%), and the inclusion of 16 patients with previous CAR T-cell therapy. The optimal partner agents for polatuzumab and its place in the DLBCL treatment algorithm remain open questions worthy of further study.
Contribution: M.N. and W.T. designed the research; M.N., W.O., D.E.-S., E.H.P., H.W.S., S.S, N.S., Y.Y.P., I.Q., J.A., R.F.M., N.P., A.K., E.D., D.W., P.M., I.K., A.C., R.K., S.C., V.S., C.B., R.A., C.W., J.C., A.B., A.A., and T.M. collected the data; W.W. analyzed the data; and M.N., W.O., C.P.F., A.J.D., C.R., and W.T. wrote the paper.
Conflict-of-interest disclosure: C.P.F served as a consultant for, had an advisory role with, and received research funding and travel grants from Roche. A.J.D. served as a consultant for or had an advisory role with Celgene, Roche, Kite Pharma, Takeda, Karyopharm Therapeutics, and Incyte; received honoraria from Celgene, Roche, Kite Pharma, Takeda, Janssen, Acerta Pharma/AstraZeneca, and ADC Therapeutics; received research funding from Celgene, Roche, Karyopharm Therapeutics, Janssen, Acerta Pharma/AstraZeneca, ADC Therapeutics, and BioInvent; and received travel grants from Celgene and Roche. D.E.-S. served as a consultant or had an advisory role with AbbVie, Astex Therapeutics, AstraZeneca, BeiGene, Janssen, and Kyowa Kirin; received honoraria from AbbVie, AstraZeneca, Janssen, Roche, and Takeda; and received travel grants from AbbVie and Novartis. N.S. served as a consultant for, had an advisory role with, and received travel grants from AbbVie, Janssen, and Roche. A.K. received honoraria from Kite Pharma, Novartis, and Celgene. P.M. served as a consultant for, had an advisory role with, or received honoraria from Roche. R.K. received honoraria from Bristol Myers Squibb and Roche. V.S. received travel grants from Gilead, Novartis, and AbbVie. R.A. served as a consultant for or had an advisory role with BeiGene and received research funding from Janssen. W.T. served as a consultant for, had an advisory role with, and received honoraria from Roche, Gilead, Celgene, and Janssen. The remaining authors declare no competing financial interests.
Correspondence: Michael Northend, University College Hospital, 3rd Floor West, 250 Euston Rd, London NW1 2PG, United Kingdom; e-mail: michael.northend@nhs.net.
References
Author notes
Requests for data sharing may be submitted to Michael Northend (michael.northend@nhs.net).
The full-text version of this article contains a data supplement.