Key Points
Treatment with pembrolizumab plus dinaciclib resulted in antitumor activity with acceptable safety for patients with rrCLL and rrDLBCL.
A careful and comprehensive approach to explore PD-1 inhibition and CDK9 inhibition in combination with other agents is needed.
Abstract
Preclinical data demonstrated that combining an anti–programmed cell death 1 (PD-1) inhibitor with a cyclin-dependent kinase 9 (CDK9) inhibitor provided enhanced antitumor activity with no significant toxicities, suggesting this combination may be a potential therapeutic option. The multicohort, phase 1 KEYNOTE-155 study evaluated the safety and antitumor activity of the PD-1 inhibitor pembrolizumab plus the CDK9 inhibitor dinaciclib in patients with relapsed or refractory (rr) chronic lymphocytic leukemia (CLL), diffuse large B-cell lymphoma (DLBCL) and multiple myeloma (MM). Patients enrolled were ≥18 years of age with a confirmed diagnosis of CLL, DLBCL, or MM. The study included 2 phases: a dose-evaluation phase to determine dose-limiting toxicities and a signal-detection phase. Patients received pembrolizumab 200 mg every 3 weeks plus dinaciclib 7 mg/m2 on day 1 and 10 mg/m2 on day 8 of cycle 1 and 14 mg/m2 on days 1 and 8 of cycles 2 and later. Primary endpoint was safety, and a key secondary endpoint was objective response rate (ORR). Seventy-two patients were enrolled and received ≥1 dose of study treatment (CLL, n = 17; DLBCL, n = 38; MM, n = 17). Pembrolizumab plus dinaciclib was generally well tolerated and produced no unexpected toxicities. The ORRs were 29.4% (5/17, rrCLL), 21.1% (8/38, rrDLBCL), and 0% (0/17, rrMM), respectively. At data cutoff, all 72 patients had discontinued treatment, 38 (52.8%) because of progressive disease. These findings demonstrate activity with combination pembrolizumab plus dinaciclib and suggest that a careful and comprehensive approach to explore anti–PD-1 and CDK9 inhibitor combinations is warranted. This trial was registered at www.clinicaltrials.gov as NCT02684617.
Introduction
Targeted therapies, including Bruton tyrosine kinase inhibitors (ibrutinib and acalabrutinib), BCL2 inhibitors (venetoclax), and phosphatidylinositol 3-kinase inhibitors (idelalisib and duvelisib), have been clinically effective in patients with chronic lymphocytic leukemia (CLL).1 However, relapse during or after initial therapy is common, warranting alternative treatment options, among them targeted immunotherapy.
Interactions between programmed cell death 1 (PD-1) receptor and its ligand PD-L1 play a critical role in tumor immune evasion. Anti–PD-1 therapy has demonstrated efficacy across several cancer types, making it an important approach for treating hematologic malignancies.2 In addition, cyclin-dependent kinase (CDK)-mediated regulation of transcription and cell cycle progression represents another attractive target for treating hematologic cancers, which are usually more sensitive to inhibition of cellular proliferation and apoptosis induction.3
Preclinical studies including murine syngeneic and human xenograft studies have demonstrated that inhibitors of CDK9 effect durable and even curative responses of lymphoma-bearing mice, which is associated with the transcriptional reduction of short-lived oncogenic MYC and antiapoptotic myeloid cell leukemia 1 (MCL1) RNA and protein expression.4,5 Furthermore, preclinical studies from Hossain et al demonstrated synergystic antitumor activity of the combination of anti–PD-1 therapy plus dinaciclib vs the single-arm controls in immunocompetent but not immunocompromised murine recipients when transplanted with MC38 tumors expressing PD-L1.6 Mechanistic studies revealed that dinaciclib improves tumor immune response through increased T-cell proliferation and dendritic cell activation, thus inducing immunogenic cell death and boosting the effects of PD-1 blockade.6
As monotherapy, the PD-1 inhibitor pembrolizumab has demonstrated high response rates and durable activity in relapsed or refractory (rr) classical Hodgkin lymphoma (cHL) and primary mediastinal large B-cell lymphoma.7,8 In contrast, dinaciclib, a CDK1, CDK2, CD5, and CDK9 inhibitor, provided modest clinical responses as a single agent in CLL, acute myeloid leukemia, acute lymphoid leukemia, multiple myeloma (MM), and diffuse large B-cell lymphoma (DLBCL).9-12 These studies, however, were not pharmacokinetically optimized for targeting of short-lived oncogenic MYC and MCL1, with infrequent dosing schedules to maximum tolerated dose. Despite this, transient marked responses including reduction in circulating blast numbers and tumor lysis syndromes in leukemias were observed with progression prior to subsequent cycles of treatment.
Proof of concept of combinatorial potential of pembrolizumab with other agents in hematological malignancy was provided by the phase 2 clinical trial of pembrolizumab in combination with pomalidomide and dexamethasone for rrMM, wherein responses were enriched for those with enhanced T-cell infiltration or higher PD-L1 expression,13 thus providing a rationale suggesting the synergistic mechanism observed in the Hossein preclinical studies may translate to efficacy in the clinic.
The multicenter, open-label, nonrandomized phase 1b KEYNOTE-155 (NCT02684617) study evaluated the safety and efficacy of pembrolizumab plus dinaciclib in patients with rrCLL, rrDLBCL, and rrMM. We present the final analysis of safety and efficacy in patients from all disease cohorts.
Methods
This study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki, and the study protocol was approved by institutional review boards or ethics committees at all participating sites. Before enrollment, all patients provided written informed consent to participate. All authors had access to the primary clinical trial data. Enrollment into the study was closed before the COVID-19 pandemic, but survivor follow-up occurred during the pandemic. Study conduct, monitoring, and oversight continued during the pandemic, and a risk-based approach was used to assess and mitigate the impact of the pandemic on study conduct.
Inclusion criteria
Eligible patients were men or women ≥18 years of age on the day they provided informed consent and who had a confirmed diagnosis of one of the following: CLL as defined by International Workshop on CLL guidelines (2008) and ≥1 previous therapy; active MM with measurable disease defined as serum monoclonal protein ≥0.5 g/dL, urine monoclonal protein ≥200 mg per 24 hours, or abnormal serum-free light chain ratio and ≥2 previous therapies, either alone or in combination with an immunomodulatory drug (ie, pomalidomide, lenalidomide, or thalidomide) and/or a proteasome inhibitor (ie, bortezomib or carfilzomib) alone or in combination; DLBCL with measurable disease defined as ≥1 lesion of >15 mm in the longest diameter and >10 mm in the short axis, disease progression after ≥2 previous therapies, including autologous stem cell transplant or ineligibility for autologous stem cell transplant. Patients had an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1, had cardiac function suitable for hydration guidelines, were able to provide newly obtained lymph node or bone marrow biopsy at screening or within 3 months of screening without intervening therapy, had confirmed disease characteristics, and had adequate organ function.
Patients with prior participation in a study with an investigational agent or device within 28 days of treatment initiation; treatment with an anticancer monoclonal antibody within 4 weeks of treatment initiation; prior allogeneic stem cell transplant within 5 years; or prior treatment with anti–PD-1/PD-L1, anticytotoxic T-lymphocyte–associated antigen-4 antibody, or a CDK inhibitor were excluded from the study. The study was performed prior to routine standard-of-care use of daratumumab (myeloma), venetoclax (CLL), or chimeric antigen receptor T-cell transplantation (DLBCL).
Study design
The study included 2 phases: a dose-evaluation phase and a signal-detection phase. Three dinaciclib dose levels ([DL]; 7 mg/m2, 10 mg/m2, and 14 mg/m2) were included in the study. Dosing schedules analyzed in the study were DL-0 (7 mg/m2, 10 mg/m2, and 14 mg/m2), DL-1 (7 mg/m2, 10 mg/m2, and 10 mg/m2), and DL-2 (7 mg/m2, 7 mg/m2, and 7 mg/m2). The dose-evaluation phase evaluated the safety of the combination of pembrolizumab plus dinaciclib at DL-0. If ≥5 of 12 patients had dose-limiting toxicity (DLT) in the dose-evaluation phase, the dinaciclib dose would be deescalated, and the modified toxicity probability interval design14 would be used to evaluate DL-0, DL-1, and DL-2 for an additional 12 patients, totaling ≤24 patients overall. Expansion cohorts were opened for the signal‐detection phase if ≤4 of the first 12 DLT–evaluable patients experienced DLTs. Each treatment cycle lasted 21 days. In the signal-detection phase, patients were enrolled in separate cohorts depending on their disease (rrCLL, rrMM, or rrDLBCL). The dose-evaluation phase and the signal-detection phase evaluated safety and preliminary efficacy outcomes in all patients who received ≥1 dose of pembrolizumab plus dinaciclib.
DLTs were defined from toxicities observed during cycles 1 and 2 if judged by the investigator to be possibly, probably, or definitely related to pembrolizumab or dinaciclib and were considered the following: grade 4 nonhematologic toxicity (not laboratory); grade 4 hematologic toxicity lasting >7 days, except thrombocytopenia (grade 4 thrombocytopenia of any duration or if grade 3 thrombocytopenia is associated with bleeding); any grade 3 nonhematologic toxicity (not laboratory), with the exception of grade 3 nausea, vomiting, or diarrhea, which will not be considered a DLT unless lasting more than 3 days despite optimal supportive care; any grade 3 or grade 4 nonhematologic laboratory abnormality if medical intervention is required or the abnormality leads to hospitalization, or the abnormality persists for >1 week; febrile neutropenia grade 3 or grade 4; any drug-related adverse event (AE) which caused subject to discontinue treatment during cycle 1 or cycle 2; grade 5 toxicity; any treatment-related toxicity which causes a >2 week delay in initiation of cycle 2 or cycle 3. Patients with a reported AE of malignant neoplasm progression will be discontinued from study treatment either on their own or by physician decision with documented disease progression based on study-specified response criteria.
Patients received pembrolizumab 200 mg by IV infusion on day 1 of each 21-day cycle plus dinaciclib 7 mg/m2 IV on day 1 and 10 mg/m2 on day 8 of cycle 1, then 14 mg/m2 IV on days 1 and 8 of cycles 2 and later. Treatment continued for up to 35 cycles or until disease progression or unacceptable toxicity.
Treatment assessments
AEs were collected throughout the study and for 30 days thereafter (90 days for serious AEs) and were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
Tumor response was assessed every 12 weeks for rrCLL and rrDLBCL and at the beginning of each cycle for rrMM by investigator review based on International Workshop on CLL guidelines (2008) for the rrCLL cohort,15 revised International Working Group response criteria for malignant lymphoma (2007) for the rrDLBCL cohort,16 and International Myeloma Working Group Uniform Response Criteria (2006) for the rrMM cohort.17
Patients continued treatment of 35 cycles (∼2 years) or until disease progression or unacceptable toxicity. Patients who discontinued treatment before disease progression, including patients who discontinued treatment of bone marrow transplant or to receive any other treatment, were followed up every 12 weeks until disease progression was documented.
In the dose-evaluation phase, at least 3 patients in each disease group were enrolled in the initial cohort of 12. Safety analysis was performed after the 12 patients in the dose-evaluation cohort received ≥2 cycles of treatment, discontinued treatment, or died of treatment-related toxicity. An interim analysis was performed for each signal-detection cohort after those 12 patients underwent their first disease response assessment.
Endpoints
Primary endpoints included safety and tolerability in both phases. Secondary endpoints for both phases included objective response rate (ORR), duration of response (DOR), and progression-free survival (PFS) for each disease type per investigator assessment and overall survival (OS).
In the signal-detection phase, a futility analysis was conducted for patients in each respective disease subgroup after the first 12 patients received ≥1 post–baseline disease assessment, and the ORR was evaluated per disease-specific criteria. Enrollment was paused for each disease cohort during the futility evaluation. If 0 responses in rrMM and rrDLBCL cohorts or ≤2 responses in the rrCLL cohort were observed among the first 12 patients, the respective cohort would be terminated. When the rrMM cohort was closed to enrollment on 7 August 2017, because of lack of efficacy, all patients with rrMM were discontinued from treatment. All remaining participants proceeded to survival follow-up. When the rrCLL cohort was closed to enrollment on 30 April 2019, because of lack of accrual, all participants with rrCLL were discontinued from treatment. All remaining participants proceeded to survival follow-up. The rrDLBCL cohort completed enrollment.
Statistical analysis
In the dose-evaluation and signal-detection phases, safety and tolerability were assessed by clinical review of all relevant parameters, including AEs, events of clinical interest, laboratory tests, and vital signs.
The primary analysis population for efficacy and safety assessments was the all-subjects-as-treated population, defined as all participants who received ≥1 dose of study treatment (pembrolizumab or dinaciclib). The point estimate and a 2-sided exact 95% confidence interval (CI) was calculated for ORR. DOR, PFS, and OS were analyzed using the Kaplan-Meier method.
Results
Seventy-two patients were enrolled and received ≥1 dose of study treatment (rrCLL cohort, n = 17; rrDLBCL cohort, n = 38; rrMM cohort, n = 17) between May 2016 and March 2019. The median age of patients was 65 years (range, 39‐85); 40 (55.6%) had ECOG PS of 1, and 47 (65.3%) had previously received ≥3 therapies (Table 1). Eighteen patients (25.0%) previously received ≥5 therapies. Sixteen patients (21.9%) received bortexomib and 18 patients (24.7%) received lenalidomide prior to enrollment (Table 1). No patients with DLBCL received chimeric antigen receptor therapy. The median number of days (range) for combination treatment was 50 (1-492) for the rrCLL cohort, 51 (1-471) for the rrDLBCL cohort, and 29 (8-71) for the rrMM cohort. Patients received a median of 3, 3, and 2 doses of pembrolizumab plus dinaciclib, respectively, for the rrCLL, rrDLBCL, and rrMM cohorts (Table 1). By data cutoff (5 May 2020), all patients discontinued treatment: 38 (52.8%) because of progressive disease (PD) (8 [47.1%] rrCLL, 21 [55.3%] rrDLBCL, 9 [52.9%] rrMM), 21 (29.2%) because of physician decision, 5 (6.9%) because of patient decision, and 8 (11.1%) because of AEs.
Twelve patients were enrolled in the dose-evaluation phase, comprised of 3 patients each in the rrCLL and rrMM disease subtype cohort and 6 patients in the rrDLBCL cohort. Of the 12 patients, DLTs were observed in 3 patients; 1 patient in the rrCLL disease subgroup had 2 DLTs (grade 4 nonserious neutropenia and grade 4 serious pulmonary sepsis, both resolved), 1 patient in the rrDLBCL disease subgroup had 1 DLT (grade 3 serious tumor lysis syndrome, resolved), and 1 patient in the rrMM disease subgroup had 1 DLT (grade 4 nonserious neutropenia, resolved with sequelae). Pembrolizumab 200 mg on day 1 of each cycle coadministered with dinaciclib (7 mg/m2 and 10 mg/m2 days 1 and 8 of cycle 1 and 14 mg/m2 in cycles 2 and later) showed an acceptable safety profile that allowed further exploration of this dose in expansion cohorts. Sixty patients were enrolled in the signal-detection phase (rrCLL, n = 14; rrDLBCL, n = 32; rrMM, n = 14).
Seventy patients (97.2%) experienced ≥1 any-grade AE, and 54 patients (75%) experienced grade 3 to 5 AEs (Table 2). The most common any-grade AEs (incidence ≥20%) in the total population were fatigue (40.3%), nausea (36.1%), anemia (27.8%), constipation (27.8%), diarrhea (22.2%), decreased platelet count (22.2%), and cough (20.8%). Forty-six of 72 (63.9%) patients experienced ≥1 TRAE (Table 2), usually decreased neutrophil count (19.4%), nausea (16.7%), fatigue (15.3%), or decreased platelet count (15.3%) (supplemental Table 1). Twenty-eight patients (38.9%) experienced grade 3 to 5 TRAEs, usually decreased neutrophil (18.1%), platelet (9.7%), or lymphocyte (6.9%) count (supplemental Table 1). Twelve patients (16.7%) experienced ≥1 treatment-related serious AE, and 8 patients (11.1%) experienced grade 3 to 4 treatment-related serious AEs. TRAEs led to discontinuation in 3 patients (4.2%). Three patients died of AEs considered by the investigators not to be related to study treatment: 1 in the CLL cohort (pneumonia) and 2 in the rrDLBCL cohort (1 of unknown cause ≤90 days after last study treatment and 1 of sepsis). No treatment-related deaths were reported in any disease cohorts (Table 2). Immune-related AEs and infusion-related AEs occurred in 6 patients (8.3%) (1 each with hypothyroidism, hyperthyroidism, or pneumonitis; 3 with infusion reactions) (supplemental Table 2).
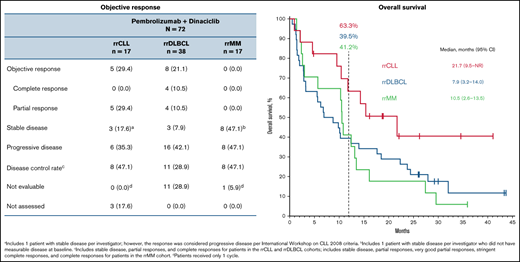
The ORR was 29.4% (95% CI, 10.3-56.0) (0 complete response, 5 partial response) for the rrCLL cohort and 21.1% (95% CI, 9.6-37.3) (4 complete response, 4 partial response) for the rrDLBCL cohort (Table 3). Median DOR was 10.3 months (range, 2.7+ to 11.1) and 4.9 months (range, 2.1 to 23.7+), respectively (Figure 1; Table 3). Three patients in the rrDLBCL cohort had a response of ≥12 months. No patients in the rrMM cohort responded (Figure 1; Table 3).
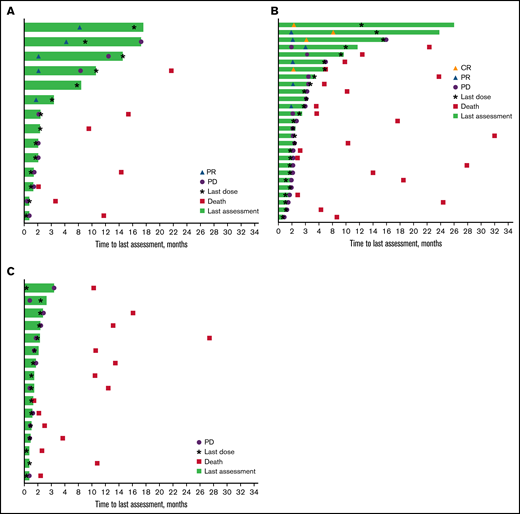
Time on study and response duration* per investigator assessment. Patients with rrCLL (A), rrDLBCL (B), and rrMM (C). CR, complete response; PR, partial response. *Tumor response as assessed by investigator review according to International Workshop on CLL guidelines (2008) for the rrCLL cohort, revised International Working Group response criteria for malignant lymphoma (2007) for the rrDLBCL cohort, and International Myeloma Working Group Uniform Response Criteria (2006) for the rrMM cohort.
Time on study and response duration* per investigator assessment. Patients with rrCLL (A), rrDLBCL (B), and rrMM (C). CR, complete response; PR, partial response. *Tumor response as assessed by investigator review according to International Workshop on CLL guidelines (2008) for the rrCLL cohort, revised International Working Group response criteria for malignant lymphoma (2007) for the rrDLBCL cohort, and International Myeloma Working Group Uniform Response Criteria (2006) for the rrMM cohort.
The majority of responders in the rrCLL and rrDLBCL cohorts were males (7 of 8 patients [80.0%] and 4 of 5 patients [87.5%], respectively) and all were age ≥65 years. A higher proportion of responders in the rrDLBCL cohort compared with those in the rrCLL cohort had an ECOG PS of 1 (62.5% vs 40.0%) and all had ≥2 prior lines of therapy (100.0% vs 40.0%). In comparing responders with rrDLBCL to all patients included in the rrDLBCL cohort, the predominant DLBCL disease subtype was activated B-cell type for both.
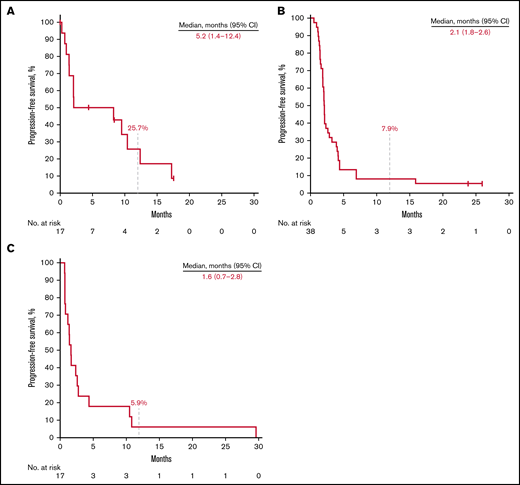
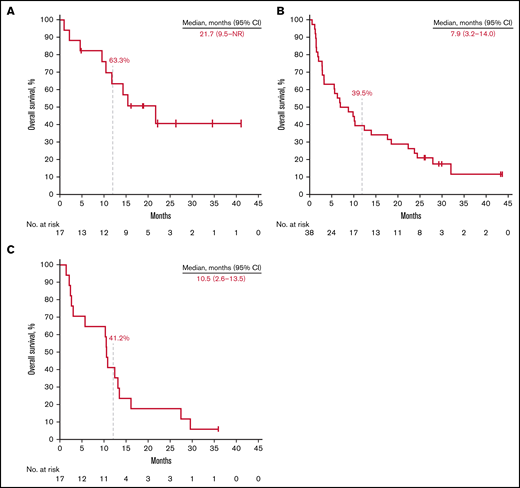
Thirteen patients (76.5%) in the rrCLL cohort, 36 (94.7%) in the rrDLBCL cohort, and 17 (100%) in the rrMM cohort experienced disease progression or death. Median PFS was 5.2 months (95% CI, 1.4-12.4) in the rrCLL cohort, 2.1 months (95% CI, 1.8-2.6) in the rrDLBCL cohort, and 1.6 months (95% CI, 0.7-2.8) in the rrMM cohort. Twelve-month PFS rates were 25.7%, 7.9%, and 5.9%, respectively (Figure 2; Table 3). Nine patients (52.9%) in the rrCLL cohort, 32 (84.2%) in the rrDLBCL cohort, and 16 (94.1%) in the rrMM cohort died. Median OS was 21.7 months (95% CI, 9.5-NR) in the rrCLL cohort, 7.9 months (95% CI, 3.2-14.0) in the rrDLBCL cohort, and 10.5 months (95% CI, 2.6-13.5) in the rrMM cohort. Twelve-month OS rates were 63.3%, 39.5%, and 41.2%, respectively (Figure 3; Table 3).
Kaplan-Meier estimates of PFS. Patients with rrCLL (A), rrDLBCL (B), and rrMM (C).
Kaplan-Meier estimates of PFS. Patients with rrCLL (A), rrDLBCL (B), and rrMM (C).
Kaplan-Meier estimates of overall survival. Patients with relapsed or refractory rrCLL (A), rrDLBCL (B), and rrMM (C).
Kaplan-Meier estimates of overall survival. Patients with relapsed or refractory rrCLL (A), rrDLBCL (B), and rrMM (C).
Discussion
In KEYNOTE-155, pembrolizumab plus dinaciclib had a manageable safety profile as evidenced by relatively low proportion of discontinuation related to TRAEs as compared with incidence of AEs. Limited efficacy of treatment was observed in a treatment-refractory population of patients with advanced hematologic malignancies. DLT incidence was low, allowing for clear signal detection for expansion cohorts. The combination demonstrated a safety profile consistent with that observed for pembrolizumab and dinaciclib as single agents in other cancers. Despite most patients experiencing ≥1 AE, no additional safety concerns or TRAE-related deaths were reported. No new immune-mediated events were associated with pembrolizumab plus dinaciclib.
Prior antitumor experience with anti–PD-1 and dinaciclib alone or in combination have been inconsistent overall in non-Hodgkin lymphoma (NHL) and MM. In the phase 1b KEYNOTE-013 study in patients with rrMM (N = 30), pembrolizumab monotherapy was unable to produce any objective response (PR or CR) after 20 months (median) of follow-up.18 In the phase 1/2 study by Kumar et al, in patients with rrMM and ≤5 prior lines of therapy (N = 27), ORR (PR or better) was 11% (3 of 27 patients) after 15 months (median) of follow-up.11 Dinaciclib monotherapy for rrCLL associated with a modest overall response rate of 54% (28 of 52 patients) in the phase 1 study by Flynn et al.9
Ribrag et al noted that combination therapy with pomalidomide and dexamethasone may enhance anti–PD-1 efficacy in rrMM.18 However, studies of combination therapies in rrMM are associated with poor overall response rates.19-21 In the phase 2 CheckMate 436 study in patients with (rr) primary mediastinal large B-cell lymphoma (PMBCL) (N = 30), combination therapy with nivolumab plus brentuximab vedotin demonstrated an ORR of 73% after 11 months of follow-up.22 Furthermore, in a phase 1b study of combination nivolumab and anti–CTLA-4 or killer-cell immunoglobulin-like receptor blockade in patients with rrcHL, rrNHL, or rrMM (N = 65), ORR ranged from 9% to 22% in rrNHL and, as expected, no response was seen in patients with rrMM.21
In the current study, combination therapy did not demonstrate efficacy in rrMM but showed modest efficacy in rrCLL and rrDLBCL, similar to prior experience with each agent alone or in combination. ORRs for rrCLL and rrDLBCL were similar to prior experience with combination therapies in NHL but not with cHL or PMBCL.21 Efficacy results were immature in patients with rrCLL because enrollment for this cohort was closed early given lack of accrual. Enrollment into the rrMM cohort was closed early due to lack of efficacy. All patients discontinued treatment by the data cutoff date, with more than half the total population experiencing PD, possibly limiting the clinical activity seen in the rrCLL and rrDLBCL cohorts. Early discontinuation because of PD resulted in shortened follow-up times, rendering this analysis underpowered for efficacy analysis. As such, efficacy results presented here should be interpreted with caution.
In conclusion, the observed ORRs and DORs from the rrCLL and rrDLBCL cohorts (up to 11 and 23 months, respectively) demonstrated limited antitumor activity of pembrolizumab plus dinaciclib in these disease cohorts. Despite this, these findings are of relevance within the rrDLBCL setting. Firstly, there is emerging evidence of a subset of DLBCL with genomic similarity to PMBCL/cHL according to copy number alterations affecting chromosome 9p24 with an associated T-cell inflammatory phenotype.23 Furthermore, according to concomitant somatic mutations present in that report, this entity appears to be independent from the LymphGen classification recently reported by the Staudt group.24 It is posited that these DLBCLs would be enriched for response to PD-1 inhibition as is the case for PMBCL and cHL.
Secondly, the development of more selective and more potent CDK9 inhibitors and their validation of preclinical efficacy in solid organ tumors and blood cancers is leading to renewed interest in their clinical development. The findings described herein provide a useful background with which to impact trials in progress of these more potent and more selective CDK9 inhibitors. Despite relatively high incidence of grade 3 or more AEs, discontinuation due to AEs was infrequent, and no new treatment-related safety signals were observed from the combination in comparison with AEs associated with either agent as monotherapy. These findings advocate a careful and comprehensive approach to exploring PD-1 inhibition and CDK9 inhibition in combination with other agents, particularly for treating patients with rrDLBCL.
Acknowledgments
The authors thank the patients and their families and all investigators and site personnel. They also thank Jennifer Gwo (employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA) for her statistical expertise. Medical writing and editorial assistance were provided by Matthew Grzywacz of ApotheCom (Yardley, PA, USA). This assistance was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
This study was supported by research funding from Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. No grant number is applicable.
Authorship
Contribution: G.P.G. conception, design or planning of the study, acquisition of the data, interpretation of the results, and critically reviewing/revising the manuscript for important intellectual content; S.K. conception, design or planning of the study, acquisition of the data, interpretation of the results, drafting of the manuscript, and critically reviewing/revising the manuscript for important intellectual content; D.W. analysis of the data, interpretation of the results, and critically reviewing/revising the manuscript for important intellectual content. D.M. acquisition and analysis of the data, interpretation of the results, and critically reviewing/revising the manuscript for important intellectual content; P.W. analysis of the data, interpretation of the results, and critically reviewing/revising the manuscript for important intellectual content; N.W.-J. acquisition of the data, interpretation of the results, and critically reviewing/revising the manuscript for important intellectual content; C.E. acquisition of the data and critically reviewing/revising the manuscript for important intellectual content; R.B. acquisition of the data, interpretation of the results, and critically reviewing/revising the manuscript for important intellectual content; D.B. acquisition of the data and critically reviewing/revising the manuscript for important intellectual content; J.C. acquisition of the data and critically reviewing/revising the manuscript for important intellectual content; F.J.H.-I. acquisition of the data and critically reviewing/revising the manuscript for important intellectual content; A.K. acquisition of the data, interpretation of the results, and critically reviewing/revising the manuscript for important intellectual content; J.M.P. acquisition and analysis of the data, interpretation of the results, and critically reviewing/revising the manuscript for important intellectual content; W.R. conception, design, or planning of the study, acquisition and analysis of the data, interpretation of the results, and critically reviewing/revising the manuscript for important intellectual content; A.J.Y. acquisition of the data, interpretation of the results, and critically reviewing/revising the manuscript for important intellectual content; A.M. acquisition of the data, interpretation of the results, and critically reviewing/revising the manuscript for important intellectual content; M.H. analysis of the data and critically reviewing/revising the manuscript for important intellectual content; M.F. acquisition and analysis of the data, interpretation of the results, and critically reviewing/revising the manuscript for important intellectual content; P.M. analysis of the data, interpretation of the results and critically reviewing/revising the manuscript for important intellectual content; and H.Q. acquisition of the data, interpretation of the results, drafting of the manuscript, and critically reviewing/revising the manuscript for important intellectual content; and all authors provided final approval to submit the manuscript.
Conflict-of-interest disclosure: G.P.G. received honoraria from Roche; is a member of the advisory boards for Roche, Janssen, Novartis/Sandoz, and Gilead; and received research funding from BeiGene, Janssen, Merck, and AbbVie. S.K. received research funding for clinical trials to the institution for AbbVie, Amgen, Bristol Myers Squibb, Carsgen, Janssen, Kite, Merck, AstraZeneca, Novartis, Roche/Genentech, Takeda, and Tenebio; is a consultant/advisory board member (no personal payments) for AbbVie, Amgen, Bristol Myers Squibb, Janssen, Roche/Genentech, Takeda, Kite, AstraZeneca, and Bluebird Bio; and is a consultant/advisory board member (with personal payment) for Oncopeptides, Beigene, and Antengene. N.W.-J. is on advisory boards for Epizyme, Karyopharm, Seattle Genetics, Grunenthal, Regeneron, ADC Therapeutics, and Verastem. R.B. received clinical trial funding (to the institution) from AbbVie, Roche, Genentech, Regeneron, and Pharmacyclics and has a family member who is an employee of Sanofi-Pasteur. D.B. received research funding from and is an advisory board member for Sanofi Pharmaceuticals. F.J.H.-I. is a consultant for and received honoraria from AstraZeneca, Amgen, Pharmacyclics, Seattle Genetics, Kite Pharmaceuticals, Curio, Novartis, and Karyopharm. J.M.P. is a consultant for AstraZeneca, Beigene, Gilead, Kite, Epizyme, Incyte, Morphosys, MEI Pharma, Actinium, WebMD, Med Learning Group, PrecisCa, and Peromius. W.R. is an advisory board member and consultant for Merck and Spark Therapeutics (Roche) (funding and personal payments). A.J.Y. is a consultant for Adaptive Biotechnologies, Amgen, Bristol Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Karyopharm, Oncopeptides, Sanofi, and Takeda and received research funding from Amgen, Bristol Myers Squibb, Celgene, and Janssen. M.H. is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. M.F. is an employee and stockholder of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. P.M. is an employee and stockholder of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. H.Q. received research funding from Amgen, Sanofi, Celgene, Bristol Myers Squibb, GlaxoSmithKline, and Karyopharm and is on the advisory boards for Amgen, Sanofi, Celgene, Bristol Myers Squibb, GlaxoSmithKline, Karyopharm, Janssen Cilag, Takeda, and CSL. The remaining authors declare no competing financial interests.
Correspondence: Gareth P. Gregory, School of Clinical Sciences at Monash Health, Monash University, 246 Clayton Rd, Clayton, VIC 3168, Australia; e-mail: gareth.gregory@monashhealth.org.
References
Author notes
Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA (MSD), is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company’s clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at: http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the US and EU or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts, after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.
The full-text version of this article contains a data supplement.