TO THE EDITOR:
Many pediatric myeloid leukemias are driven by fusion proteins resulting from unique chromosomal translocations. The most frequent chimeric oncogene in non–Down syndrome acute megakaryoblastic leukemia is the cryptic inv(16)(p13.3q24.3) translocation, which encodes the CBFA2T3-GLIS2 (CBF/GLIS) fusion protein. The CBF/GLIS fusion protein promotes aggressive megakaryoblastic leukemia (AML) and other myeloid leukemias that are exclusive to infants and young children.1-5 Patients with this fusion are refractory to conventional therapy, frequently relapsing after myeloablative stem cell transplantation. The 5-year survival rates range between 15% and 30%.5,6
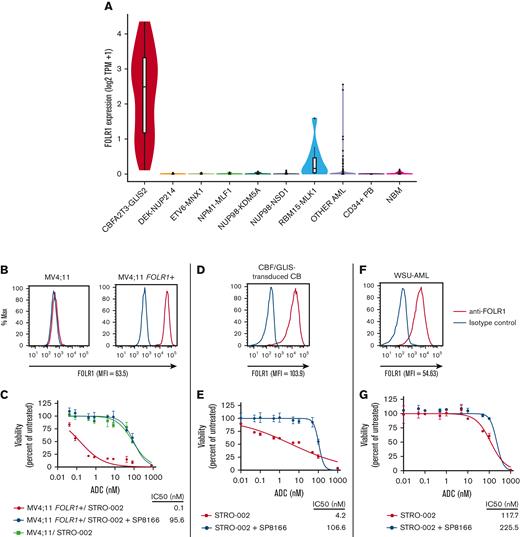
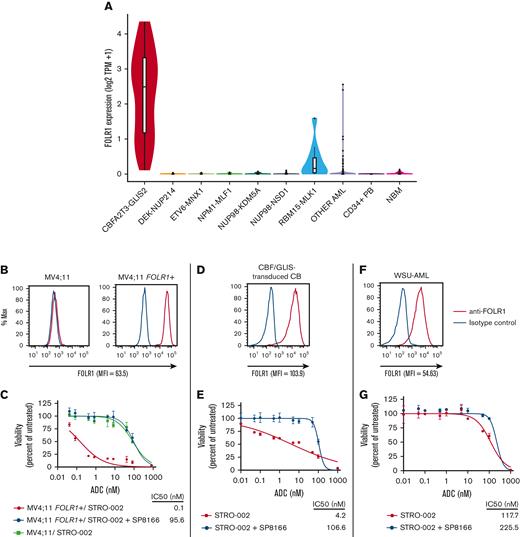
To identify therapeutic targets for CBF/GLIS AML, we interrogated the transcriptome of more than 2000 AML cases, including 38 cases of CBF/GLIS AML, for AML-specific gene targets. We identified the FOLR1 transcript to be uniquely expressed in CBF/GLIS AML but silent in most other types of AML, normal CD34+ cells, and bone marrow samples (Figure 1A). FOLR1 encodes folate receptor α and is also overexpressed in many solid tumors.7 We have previously demonstrated cell surface expression of FOLR1 in primary CBF/GLIS patient samples.8 In addition, we also showed that cord blood cells that were transduced with a CBF/GLIS expression construct acquired high levels of FOLR1 expression, suggesting that FOLR1 expression is induced by the CBF/GLIS fusion oncoprotein.8 Thus, FOLR1 may provide a promising and specific antigen target for CBF/GLIS AML. Here, we present preclinical studies on the efficacy of STRO-002, a FOLR1-directed antibody-drug conjugate (ADC), developed by Sutro Biopharma for solid malignancies,9 against CBF/GLIS AML.
FOLR1 is uniquely expressed in CBF/GLIS AML, and FOLR1-expressing AML is sensitive to STRO-002, a FOLR1-directed ADC. (A) Box/violin plot of FOLR1 transcript expression in CBF/GLIS AML (N = 38), DEK-NUP214 (N = 48), ETV6-MNX1 (N = 16), NPM1-MLF1 (N = 8), NUP98-KDM5A (N = 32), NUP98-NSD1 (N = 104), RBM15-MKL1 (N = 10), other AML (N = 1235), normal peripheral blood CD34+ samples (N = 16), and normal bone marrow samples (N = 68) from TARGET Pediatric AML study. (B,D,F) Flow cytometric analysis of FOLR1 cell surface expression on MV4;11 parental cells and MV4;11 cells transduced with FOLR1 expression construct (MV4;11 FOLR1+), CBF/GLIS-transduced CB hematopoietic stem/progenitor cells (HSPCs) and WSU-AML cells, respectively. The FOLR1 mean fluorescent intensity (MFI) vs isotype is shown at the bottom of the histogram. (C,E,G) In vitro cytotoxicity of STRO-002 against MV4;11 and MV4;11 FOLR1+, CBF/GLIS-transduced cord blood (CB) HSPCs and WSU-AML cells, respectively. Cells were treated with increasing doses of STRO-002 alone or in excess of naked antibody SP8166 (1 uM). After 3 days of continuous exposure, viability was assessed by Cell Titer-Glo assay. Data are normalized to untreated controls. Error bars denote standard deviation from 2 technical replicates at each dose. Max, maximum; NBM, normal bone marrow; TPM, Transcripts per million.
FOLR1 is uniquely expressed in CBF/GLIS AML, and FOLR1-expressing AML is sensitive to STRO-002, a FOLR1-directed ADC. (A) Box/violin plot of FOLR1 transcript expression in CBF/GLIS AML (N = 38), DEK-NUP214 (N = 48), ETV6-MNX1 (N = 16), NPM1-MLF1 (N = 8), NUP98-KDM5A (N = 32), NUP98-NSD1 (N = 104), RBM15-MKL1 (N = 10), other AML (N = 1235), normal peripheral blood CD34+ samples (N = 16), and normal bone marrow samples (N = 68) from TARGET Pediatric AML study. (B,D,F) Flow cytometric analysis of FOLR1 cell surface expression on MV4;11 parental cells and MV4;11 cells transduced with FOLR1 expression construct (MV4;11 FOLR1+), CBF/GLIS-transduced CB hematopoietic stem/progenitor cells (HSPCs) and WSU-AML cells, respectively. The FOLR1 mean fluorescent intensity (MFI) vs isotype is shown at the bottom of the histogram. (C,E,G) In vitro cytotoxicity of STRO-002 against MV4;11 and MV4;11 FOLR1+, CBF/GLIS-transduced cord blood (CB) HSPCs and WSU-AML cells, respectively. Cells were treated with increasing doses of STRO-002 alone or in excess of naked antibody SP8166 (1 uM). After 3 days of continuous exposure, viability was assessed by Cell Titer-Glo assay. Data are normalized to untreated controls. Error bars denote standard deviation from 2 technical replicates at each dose. Max, maximum; NBM, normal bone marrow; TPM, Transcripts per million.
To evaluate the specificity of STRO-002 against FOLR1-positive AML, MV4;11 AML cells were transduced with a FOLR1-expressing construct (MV4;11 FOLR1+; Figure 1B). The viability of MV4;11 FOLR1+ cells and parental MV4;11 cells lacking FOLR1 expression was determined after incubation with increasing concentrations of STRO-002. Compared with parental MV4;11 cells, MV4;11 FOLR1+ cells were highly susceptible to STRO-002–mediated cytotoxicity (half-maximal inhibitory concentration [IC50]: 0.1 nM vs 86.6 nM; Figure 1C). Similar potency was observed in the FOLR1-expressing IGROV-1 ovarian cancer cell line (IC50: 3.8 nM; supplemental Figure 1). The response was specific to FOLR1; incubation with an excess unconjugated anti-FOLR1 monoclonal antibody, SP8166, inhibited cytotoxicity of STRO-002 in MV4;11 FOLR1+ (Figure 1B) and IGROV-1 cells (supplemental Figure 1) but had no effect on parental MV4;11 cells.
Then, we evaluated the preclinical efficacy of STRO-002 in CBF/GLIS AML, using CBF/GLIS-transduced CB HPSCs (CBF/GLIS-CB), a cell line we previously generated that models the molecular and cellular properties of primary CBF/GLIS AML8 and WSU-AML, a CBF/GLIS AML cell line with endogenous expression of FOLR1. Consistent with previous studies, CBF/GLIS-CB demonstrated bright cell surface expression of FOLR1 by using flow cytometry (Figure 1D). We incubated CBF/GLIS-CB cells with increasing concentrations of STRO-002 alone or in excess amounts of the blocking antibody (SP8166). After a 5-day incubation, STRO-002 therapy induced significant dose-dependent cytotoxicity against CBF/GLIS-CB cells (IC50: 4.2 nM) compared with cells incubated with blocking antibody (IC50: 106.6 nM; Figure 1E). In contrast, WSU-AML cells had lower expression of FOLR1 (Figure 1F), and STRO-002 had minimal effect on the viability of WSU-AML cells (Figure 1G). The lack of efficacy may be due to the lower expression of FOLR1 in WSU-AML cells (mean fluorescence intensity: WSU-AML, 54.63; CBF/GLIS-CB, 103.9; MV411 FOLR1+, 63.5; Figure 1B, D, and F) or possible effect of folate in the culture medium.
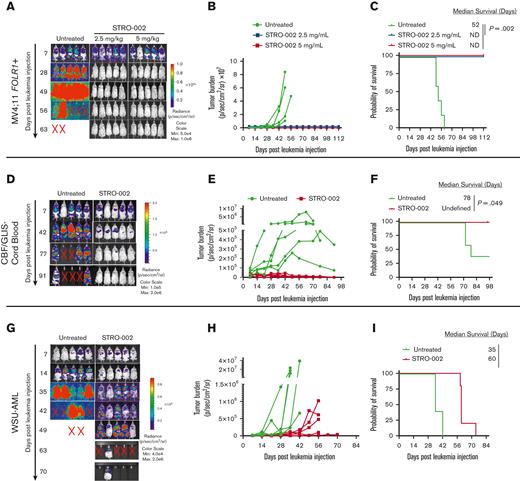
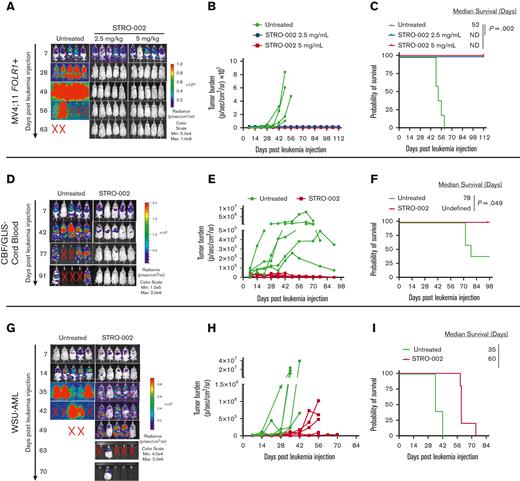
To assess the activity of STRO-002 in xenograft mouse models, we first transplanted MV4;11 FOLR1+ cells transduced with green fluorescent protein/firefly luciferase into NOD/SCID/IL2rγnull NOD scid gamma(NSG) mice. The mice that underwent transplantation received 3 weekly doses of STRO-002 at 2.5 mg/kg and 5 mg/kg 1 week after transplantation (supplemental Figure 2A). The STRO-002 treatment induced leukemia clearance to undetectable levels in both the treatment cohorts as determined by bioluminescent imaging (Figure 2A-B). In addition, STRO-002 therapy resulted in stable complete remission, with treated mice surviving up to the end of the study (112 days) compared with untreated MV4;11 FOLR1+ xenograft mice, which developed progressive disease and had a median survival of 52 days after receiving injection for leukemia (P = .002; Figure 2C).
Preclinical efficacy of STRO-002 against FOLR1-expressing AML xenograft models. (A) Leukemia burden measured by bioluminescence (in vivo imaging system) imaging in MV4;11 FOLR1+ xenograft mice untreated or treated with STRO-002 at 2.5 mg/kg or 5 mg/kg weekly for 3 weeks. Shown are representative time points. N = 5 mice per group. (B) Quantification of radiance is shown in panel A. (C) Kaplan-Meier survival curves of MV4;11 FOLR1+ xenografts untreated or treated with STRO-002. Statistical differences in survival were evaluated using log-rank Mantel-Cox. (D,G) Leukemia burden measured by bioluminescence (in vivo imaging system) imaging in CBF/GLIS-transduced CB HSPC–bearing mice or WSU-AML–bearing mice untreated or treated with STRO-002 at 10 mg/kg weekly for 3 weeks, respectively. Shown are representative time points. N = 5 mice per group. (E,H) Quantification of radiance is shown in panels D and G, respectively. (F,I) Kaplan-Meier survival curves of CBF/GLIS-transduced CB HSPC–bearing mice or WSU-AML–bearing mice, respectively, untreated or treated with STRO-002. Statistical differences in survival were evaluated using log-rank Mantel-Cox. Symbol X indicates death. ND, not detected.
Preclinical efficacy of STRO-002 against FOLR1-expressing AML xenograft models. (A) Leukemia burden measured by bioluminescence (in vivo imaging system) imaging in MV4;11 FOLR1+ xenograft mice untreated or treated with STRO-002 at 2.5 mg/kg or 5 mg/kg weekly for 3 weeks. Shown are representative time points. N = 5 mice per group. (B) Quantification of radiance is shown in panel A. (C) Kaplan-Meier survival curves of MV4;11 FOLR1+ xenografts untreated or treated with STRO-002. Statistical differences in survival were evaluated using log-rank Mantel-Cox. (D,G) Leukemia burden measured by bioluminescence (in vivo imaging system) imaging in CBF/GLIS-transduced CB HSPC–bearing mice or WSU-AML–bearing mice untreated or treated with STRO-002 at 10 mg/kg weekly for 3 weeks, respectively. Shown are representative time points. N = 5 mice per group. (E,H) Quantification of radiance is shown in panels D and G, respectively. (F,I) Kaplan-Meier survival curves of CBF/GLIS-transduced CB HSPC–bearing mice or WSU-AML–bearing mice, respectively, untreated or treated with STRO-002. Statistical differences in survival were evaluated using log-rank Mantel-Cox. Symbol X indicates death. ND, not detected.
To determine the efficacy of STRO-002 in a xenograft model of CBF/GLIS AML, we transplanted CBF/GLIS-CB cells into NSG-SGM3 mice. CBF/GLIS-CB xenograft mice were treated with STRO-002 1 week after the transplant at 10 mg/kg once a week for 3 weeks (supplemental Figure 2B). As observed with transplanted MV4;11 FOLR1+ cells, STRO-002 therapy also drastically inhibited leukemic growth driven by CBF/GLIS-CB cells. By day 49, after the leukemia injection, leukemia burden in CBF/GLIS-CB xenograft mice was undetectable by image analysis with survival to the end of the study (98 days; Figure 2D-F). In contrast, all of the mice in the no-treatment group developed leukemia, with 3 of 5 mice exhibiting symptomatic disease (median survival, 78 days; Figure 2D-F). We also tested the in vivo activity of STRO-002 against a xenograft model with WSU-AML (supplemental Figure 2C). Leukemia in WSU-AML xenograft mice was less sensitive to STRO-002 therapy. The treatment resulted in a significant but transient reduction of the disease in WSU-AML xenograft mice compared with untreated mice and increased survival from 35 to 60 days after receiving the injection for leukemia (P = .002; Figure 2G-I).
In this study, we show that FOLR1 is uniquely expressed in CBF/GLIS AML but silent in normal cells, providing a strategy to target leukemia cells without affecting normal hematopoiesis. FOLR1 is targeted by STRO-002, an ADC that is currently in a phase 1 clinical trial for the treatment of solid tumors. We demonstrate that STRO-002 exhibits target-dependent cytotoxicity against FOLR1-expressing AML cells. STRO-002 therapy is highly effective against MV4;11 FOLR1+ and CBF/GLIS-CB but has only modest activity against the CBF/GLIS-positive WSU-AML cell line expressing lower levels of FOLR1. Strategies that enhance FOLR1 expression including dexamethasone in combination with histone deacetylase inhibitors may improve the efficacy of STRO-002 against leukemias with low antigen density.10 Further studies should evaluate the effect of this combination on FOLR1 expression in CBF/GLIS AML and the activity of STRO-002.
The finding that FOLR1 is not expressed in normal hematopoietic stem/progenitor cells suggests that FOLR1 might be a specific target for CBF/GLIS AML, and FOLR1-directed immunotherapy would avoid hematopoietic toxicity. Consistent with this hypothesis, we previously showed that FOLR1-directed chimeric antigen receptor T cells do not affect cell viability, self-renewal, and multilineage differentiation of normal hematopoietic stem/progenitor cells.8 However, FOLR1 is expressed at low levels in other tissues, including the luminal epithelial surface of the kidney, lung, and choroid plexus.7 Although severe pulmonary toxicity has been reported with a FOLR1 T-cell bispecific antibody in nonhuman primates,11 ongoing phase 1 clinical trials of STRO-002 in patients with advanced ovarian cancer have shown the drug to be well tolerated without significant toxicity.9 In addition, there are many other approaches targeting FOLR1 for solid tumor therapy in different stages of development; clinical studies with these agents should provide additional data on the safety and toxicity associated with FOLR1-directed immunotherapy (www.clinicaltrials.gov).12
In conclusion, we have shown that preclinical studies with STRO-002, a FOLR1-directed ADC, can effectively eliminate CBF/GLIS-positive AML, providing a promising approach to treating this aggressive pediatric leukemia. Our studies provide compelling data to evaluate STRO-002 therapy in clinical trials for CBF/GLIS AML. In addition, our study illustrates the power of using gene expression data to identify and repurpose targeted therapies developed for the treatment of other malignancies to treat leukemias.
Acknowledgments: This study was supported by Project Stella, Target Pediatric AML, and The Leukemia & Lymphoma Society (S.M.) and Children’s Oncology Group Translation Pilot Studies for Hematopoietic Malignancies (K.R.L.).
Contribution: Q.L. designed the experiments; T.T., C.N.M., L. Perkins, L. Pardo, and S.C. performed the experiments; Q.L., T.T., and K.R.L. analyzed the data; Q.L. and K.R.L. wrote the paper; and C.A., K.B., A.M., L.E.B., M.R.L., K.T., K.R.L., and S.M. provided general guidance.
Conflict-of-interest disclosure: L. Pardo, L.E.B., and M.R.L. are employees of Hematologics Inc, and M.R.L. has equity ownership in Hematologics Inc. C.A., K.B., and A.M. are employees of Sutro Biopharma. The remaining authors declare no competing financial interests.
Correspondence: Keith R. Loeb, Clinical Research Division, Fred Hutchinson Cancer Center, 1100 Fairview Ave N, Seattle, WA 98109; e-mail: kloeb@fredhutch.org.
References
Author notes
Presented in abstract form at the 63rd annual meeting of the American Society of Hematology, Atlanta, GA, 11-14 December 2021.
The data reported in this article can be found in the Database of Genotypes and Phenotypes (accession number phs000465.v19.p8). RNA sequencing data on engineered cord blood can be found in the Gene Expression Omnibus database (accession number GSE181726).
RNA sequencing data on primary patient samples are deposited at the National Cancer Institute’s Genomic Data Commons (www.cancer.gov) under the TARGET-AML project.
Data are available on request from the corresponding author, Keith R. Loeb (kloeb@fredhutch.org).
The full-text version of this article contains a data supplement.
Q.L. and T.T. contributed equally to this study.
S.M. and K.R.L. contributed equally to this study.