Abstract
IDH2 (isocitrate dehydrogenase 2) mutations occur in approximately 15% of patients with acute myeloid leukemia (AML). The IDH2 inhibitor enasidenib was recently approved for IDH2-mutated relapsed or refractory AML. We conducted a multi-center, phase I trial of maintenance enasidenib following allogeneic hematopoietic cell transplantation (HCT) in patients with IDH2-mutated myeloid malignancies. Two dose levels, 50mg and 100mg daily were studied in a 3 × 3 dose-escalation design, with 10 additional patients treated at the recommended phase 2 dose (RP2D). Enasidenib was initiated between days 30 and 90 following HCT and continued for twelve 28-day cycles. Twenty-three patients were enrolled, of whom 19 initiated post-HCT maintenance. Two had myelodysplastic syndrome, and 17 had AML. All but 3 were in first complete remission. No dose limiting toxicities were observed, and the RP2D was established at 100mg daily. Attributable grade ≥3 toxicities were rare, with the most common being cytopenias. Eight patients stopped maintenance before completing 12 cycles, due to adverse events (n=3), pursuing treatment for graft-vs-host disease (GVHD) (n=2), clinician choice (n=1), relapse (n=1), and COVID infection (n=1). No cases of grade ≥3 acute GVHD were seen, and 12-month cumulative incidence of moderate/severe chronic GVHD was 42% (20-63%). Cumulative incidence of relapse was 16% (95% CI: 3.7-36%); 1 subject relapsed while receiving maintenance. Two-year progression-free and overall survival were 69% (95% CI: 39-86%) and 74% (95% CI, 44-90%), respectively. Enasidenib is safe, well-tolerated, with preliminary activity as maintenance therapy following HCT, and merits additional study. The study was registered at www.clinicaltrials.gov (#NCT03515512).
Key Points
Enasidenib is well-tolerated as maintenance therapy following hematopoietic cell transplantation for IDH2-mutated myeloid malignancies.
Relapse rate was low with enasidenib maintenance, with a promising 2-year progression-free and overall survival among IDH2-mutated patients.
Introduction
Outcomes for patients with acute myeloid leukemia (AML) remain poor. Allogeneic hematopoietic cell transplantation (HCT) is the lone curative modality for the majority achieving remission following initial treatment, but disease relapses are common.1,2 Characterization of molecular alterations in AML has led to development of targeted therapies,3-5 including FMS-like tyrosine kinase 3 (FLT3) and isocitrate dehydrogenase (IDH) inhibitors. Recently, such agents have shown impressive activity in clinical trials and have been approved for use, particularly in patients with relapsed/refractory (R/R) AML. These have included the FLT3 inhibitor gilteritinib, the IDH1 inhibitor ivosidenib, and the IDH2 inhibitor enasidenib.6-8
Maintenance therapy following HCT seeks to reduce the incidence of relapse and enhance the curative potential of HCT.9 This approach has recently shown substantial promise among patients with FLT3-mutated AML. Early phase clinical trials with sorafenib maintenance treatment following HCT revealed encouraging tolerability and outcomes in this historically proliferative, adverse-risk disease subtype.10,11 Randomized clinical trials of post-HCT sorafenib have since followed, demonstrating improved outcomes.12,13 A randomized phase 3 study of post-HCT gilteritinib has also completed accrual, with results eagerly awaited.
In contrast, studies assessing the role of IDH inhibitors as maintenance following HCT have been lacking. IDH2 mutations are found in ∼8% to 15% of patients with AML.14-16 Typically, unaltered IDH2 enzymes within the mitochondria play a central role in the Krebs cycle, catalyzing the conversion of isocitrate to alpha-ketoglutarate, leading to adenosine triphosphate formation and energy for the cell. The altered IDH2 proteins however, through neoenzymatic activity, catalyze the formation of the oncometabolite 2-hydroxyglutarate (2-HG) from alpha-ketoglutarate.5 2-HG has been shown to effectively suppress key enzymes involved in epigenetic regulation, including TET2 enzymes, leading to hypermethylation of DNA and histones, termination of normal myeloid differentiation, and promotion of myeloid malignancy.17
Enasidenib, a selective small molecule IDH2 inhibitor, suppresses production of 2-HG, promotes myeloid differentiation, and was associated with impressive rates of response in clinical trials.8,18 It is now approved by the Food and Drug Administration for treatment of R/R IDH2-mutated AML. The primary objective of this phase 1, multicenter, dose-finding study was to define the recommended phase 2 dose (RP2D) of enasidenib as maintenance therapy following HCT for IDH2-mutated myeloid malignancies. We also sought to gain a preliminary appreciation of the therapeutic efficacy of enasidenib in preventing disease relapse following HCT.
Methods
Patients
This multicenter study was conducted and enrolled patients at Massachusetts General Hospital, Dana-Farber Cancer Institute, and the Johns Hopkins Hospital Cancer Center. It was approved by the respective institutional review boards at these institutions. Informed consent was obtained from all patients, and the trial was conducted according to the Declaration of Helsinki. The study was registered at ClinicalTrials.gov (NCT03515512).
Eligibility included patients eligible for transplant aged 18 years and over, with AML in remission, myelodysplastic syndrome (MDS) or chronic myelomonocytic leukemia and whose disease possessed an IDH2 mutation by local testing, which could be established by polymerase chain reaction (PCR) or next-generation sequencing testing. AML in remission, for purposes of inclusion, referred to either complete remission (CR) or completion remission with incomplete hematologic recovery (CRi). CR definition was per European LeukemiaNet Criteria19 and would require marrow blasts <5%, absence of circulating blasts, absence of extramedullary disease, neutrophil count ≥1000/μL, and platelet count ≥100 000/μL. CRi was defined as all CR criteria except for residual platelet or neutrophil requirements. Conditioning eligibility could include myeloablative (MAC) or reduced-intensity (RIC) regimens, and HCT donors could be any of the following: ≥5/6 (HLA-A, B, DR), ≥7/8 (HLA-A, B, DR, C), haploidentical donor of ≥3/6 (HLA-A, B, DR), or umbilical cord blood ≥4/6 (HLA-A, B, DR). Graft-vs-host disease (GVHD) prevention was left to the discretion of the treating investigator. Patients were screened and enrolled before HCT, and they were required to have normal organ and marrow function (neutrophil count [ANC] >1000/μL and platelet count >50 000/μL). Those with prior HCT, active R/R disease, QTc ≥450 ms, and active infections were excluded. Treatment with enasidenib maintenance was initiated between day 30 and 90 following HCT, at which time, the following were required: ANC ≥ 1000/μL and platelets ≥50 000/μL, direct bilirubin ≤2.0 × upper limit of normal, chimerism ≥70% of donor origin among blood/marrow cells, no acute GVHD (aGVHD) requiring equivalent dose of ≥0.5 mg/kg per day prednisone, no disease recurrence as assessed by bone marrow biopsy, and no active infection requiring intravenous antibiotics. Use of enasidenib before HCT was allowed.
Treatment
Following HCT, enasidenib was taken once daily, by mouth, in 28-day cycles. The first dose level was 50 mg daily. The period for dose-limiting toxicity (DLT) evaluation was the first cycle, and dose escalation occurred via 2 cohorts, 50 and 100 mg daily. Dose escalation from one level to the next was guided by DLT incidence according to a standard 3 + 3 design. Following the establishment of maximum tolerated dose or RP2D, 10 patients were subsequently enrolled in an expansion cohort.
Disease status by marrow biopsy was performed before initiation of maintenance treatment, and after cycle 6 and 12. Patients were monitored for relapse and toxicity and could continue until disease progression, intolerable toxicity, or upon receipt of 12 cycles.
Correlative studies
As an assessment for measurable (or minimal) residual disease (MRD), IDH2 mutation clonal burden in the marrow was assessed for a subset of patients before HCT, and following HCT but before initiating enasidenib maintenance. During the duration of the clinical trial accrual period, IDH2 mutational burden as measured by variant allelic fraction (VAF) was measured by 3 sequential assays, with varying sensitivities. The 2 next-generation sequencing assays were performed at Brigham and Women’s Hospital – versions 2 and 3 of the Rapid Heme Panel (RHP v2 and v3). For these assays, for any given locus, the analytical sensitivity is determined by the ability to call true signal over background noise. For the IDH2 p.R140 and p.R172 loci in RHPv2, where coverage ranged from 700 to 1500× read depth, using a threshold of 10-fold above the level of noise allowed variants upon manual review to be called down to 0.2% to 0.5% VAF. RHPv3 uses unique molecular identifiers that remove most of the noise, allowing variants to be called potentially as low as 0.1% VAF at the IDH2 hotspot loci with approximately 1000× consensus read depth. A third assay for a subset of samples assessed IDH2 R140 and R172 mutation VAFs through BEAMing digital PCR technology (OncoBEAM; Sysmex Inostics, Baltimore, MD). This technique has a lower limit of detection for mutant IDH2 alleles of 0.02% to 0.04% (2 × 10−4 to 4 × 10−4). The genomic DNA isolated from the bone marrow samples met the minimum input material threshold (per assay specification) to obtain valid quantification of the fraction of mutant alleles to wild-type alleles in each sample. If the minimum threshold of DNA is satisfied, the sensitivity of the BEAMing digital PCR assay allows for accurate detection of IDH2 allelic frequencies from patient samples regardless of cellularity, including those deemed to be aplastic by the clinical investigator. Plasma samples were also collected for assessment of 2-HG levels. Plasma 2-HG concentrations were measured using a qualified liquid chromatography–mass spectrometry method with a lower limit of quantitation of 30.0 ng/mL.
Study design and endpoints
A standard 3 + 3 study design was used for dose escalation. The primary end point of this study was to establish the maximum tolerated dose or RP2D of enasidenib in the post-HCT setting. Nonhematologic DLTs were defined as any drug-related, NCI Common Terminology Criteria for Adverse Events (CTCAE v4.0) ≥ grade 3 adverse event (AE) not related to underlying disease, with exceptions being fatigue, asthenia, fever, anorexia or constipation, isolated electrolyte abnormalities that resolved to < grade 2 within 72 hours, grade 3 nausea, vomiting, or diarrhea that lasted ≤72 hours and responded to medical intervention, and amylase/lipase elevation not associated with clinical manifestations of pancreatitis. Hematologic DLTs were defined as any drug-related grade 4 neutropenia (ANC < 500/μL) or thrombocytopenia (platelets < 25 000/μL) that did not resolve to ≤ grade 1 within 14 days or resolved within 14 days but recurred after resuming enasidenib. In addition, any ≥ grade 2 nonhematologic toxicity found intolerable, or which rendered participants unable to take ≥75% of doses, febrile neutropenia of any duration or grade 3 to 4 thrombocytopenia associated with clinically significant bleeding not because of other causes, and all study drug-related deaths were considered a DLT. Participants must have received at least 50% of dosing to be DLT-evaluable. However, those receiving <50% of study drug during the first cycle, but nevertheless experienced a DLT, remained evaluable for DLT assessment.
Secondary and exploratory endpoints included cumulative rate of relapse, progression-free survival (PFS), overall survival (OS), proportion of patients who successfully screened before HCT but did not reach the maintenance phase because of HCT-related morbidity or mortality, and cumulative incidence rates of aGVHD and chronic GVHD (cGVHD), respectively. aGVHD grading was conducted as per consensus criteria, and cGVHD grading was conducted as per National Institutes of Health consensus criteria.20,21
Statistical analysis
Descriptive analysis was primarily performed. OS, PFS, and GVHD-free, relapse-free survival (GRFS) were estimated using the Kaplan-Meier method whereas cumulative incidence of aGVHD, cGVHD, relapse and nonrelapse mortality (NRM) were estimated in the competing risks framework22 treating relapse or death without developing GVHD as a competing event for GVHD, NRM for relapse and relapse for NRM as a competing event. GRFS was explored as a post hoc end point and defined as time from stem cell infusion to grade 3-4 aGVHD, moderate/severe cGVHD, relapse or death whichever occurred first. Univariable Cox regression analysis was performed to identify potential risk factors for OS and PFS. In addition, univariable Fine and Gray model was used to explore the baseline 2HG concentration in association with relapse. Although enasidenib was administered after stem cell infusion, because the trial was not intended to compare with patients that did not undergo treatment, the time 0 was set to the date of stem cell infusion for time-to-event analysis for the purpose of future reference. All testing was two-sided at the significance level of 0.05. All analyses were performed using SAS 9.3 (SAS Institute Inc, Cary, NC), and R v3.4 (the CRAN project).
Results
Patients
A total of 23 patients were registered before HCT. Of these, 4 patients did not initiate enasidenib maintenance following HCT; 1 because of withdrawal of consent, 1 because of disease relapse, 1 because of the decision of treating physician, and 1 because of logistical obstacles caused by the COVID-19 pandemic. The remaining 19 patients were initiated on treatment with enasidenib maintenance following HCT, and the median time of starting maintenance was day 55 (range 37-90) after HCT.
Demographic and clinical characteristics for these patients are provided in Table 1. The median age was 63 years (range 31-79). Twelve (63%) participants were male, and 13 (68%) were Caucasian. Seventeen patients (90%) had AML, of which 7 (41%) were AML with MDS related changes and 2 (12%) had AML arising from antecedent myeloproliferative neoplasms. Two patients (11%) had MDS-EB. Of the 17 patients with AML, 14 (82%) received intensive induction chemotherapy before HCT. Of the patients with AML, 15 (88%) received 1 line of therapy before HCT, and 2 (12%) received 2 prior lines. Both patients with MDS-EB received 2 prior lines of therapy before HCT. Among all patients receiving maintenance therapy, 10 of 19 (53%) had received enasidenib before HCT. Four patients (21%) received myeloablative and 15 (79%) received reduced-intensity conditioning before HCT. Ten patients (53%) underwent an 8/8 matched unrelated, 7 (37%) haploidentical, 1 (5.3%) matched related, and 1 (5.3%) 7/8 matched unrelated donor HCT.
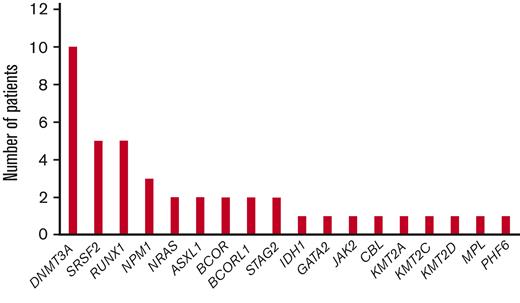
Among the 17 patients with AML, 14 (82%) had intermediate-risk cytogenetics, with 11 (65%) having a normal karyotype. Three patients (18%) had adverse-risk cytogenetics, 1 with deletion 7, 1 with complex karyotype, and 1 with an 11;21 translocation. The 2 patients with MDS had intermediate-risk karyotype. Concurrent mutational data were available for 16 of 17 patients with AML, and the most common mutations were DNMT3A (41%), SRSF2 (24%), RUNX1 (24%), and NPM1 (18%). One patient with MDS had concurrent RUNX1, SRSF2, BCOR, and BCORL1 mutations, and the other had a concurrent DNMT3A mutation (Figure 1). Among all 19 patients, 18 had available IDH2 subtype data; IDH2 R140Q were seen in 10 (53%), R172K in 7(37%), and R172G in 1 patient (5.3%) (Table 1).
Enasidenib treatment
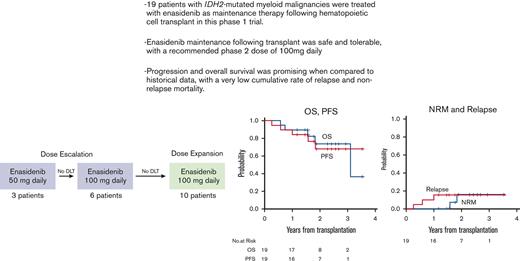
Three patients were enrolled at the 50 mg daily (the first dose level), and 6 patients at 100 mg daily dose levels for enasidenib as maintenance. No DLTs were detected, and 10 additional patients were enrolled in an expansion cohort at 100 mg daily, the RP2D. Grade 3 or higher AEs that were attributable to study treatment (either possibly, probably, or definitively) are provided in Table 2. These included anemia (2 patients, both grade 3), neutropenia (1 patient, grade 4), bilirubinemia (1 patient, grade 3), coronary ischemia (1 patient, grade 3), fatigue (1 patient, grade 3), and hyponatremia (1 patient, grade 3). Eight patients (42%) required in total 9 episodes of dose interruption, lasting a median of 24 days (range 7-29), with 6 because of treatment-related toxicity and the remainder due to concurrent GVHD. Six (32%) patients underwent a dose reduction to 50 mg, on which they continued treatment. None of these dose reductions occurred during the first cycle. In total, 8 patients (42%) discontinued study treatment before reaching 12 cycles; 3 because of AEs (bilirubinemia, coronary ischemia, elevated alkaline phosphatase), 1 because of relapse, 1 because of concurrent complications of covid infection, 1 because of clinician choice, and 2 to pursue therapies for cGVHD. The median number of completed cycles was 12 (range 2, 12 cycles). Eleven patients received all 12 planned cycles of enasidenib maintenance. For the remaining patients, 2 received 11, 1 received 7, 1 received 6, and 4 others received <6 cycles of enasidenib maintenance.
Transplant outcomes
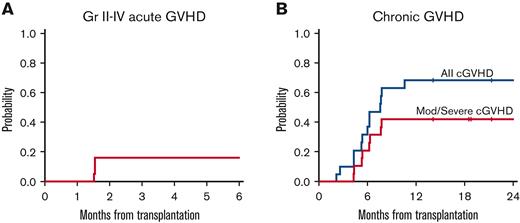
Five patients (26%) experienced aGVHD in the follow-up period after the initiation of maintenance, 3 grade 1 and 2 grade 2 cases. Five cases of aGVHD occurred before start of maintenance therapy, and therefore in total, 10 patients (52%) experienced grades 1-2 aGVHD on study, of which 7 were classified as grade 1 and 3 as grade 2. No patients experienced grade 3 or higher aGVHD. Thirteen cases of cGVHD were reported; 5 mild, 7 moderate, and 1 severe. Five patients with cGVHD required initiation of systemic steroids. The 6-month cumulative incidence of grades 2-4 aGVHD was 16% (95% confidence interval [CI], 3.7-36). The 1-year cumulative incidence of cGVHD was 68% (95% CI, 41-85), and the 1-year cumulative incidence of moderate/severe cGVHD was 42% (95% CI, 20-63) (Table 3; Figure 2). There was not a substantial change in chimerism, by T cell and bone marrow/peripheral blood analysis, before maintenance treatment and by the end of treatment (median of 99% vs 100%).
Incidence of graft-vs-host disease. (A) cumulative incidence of grade II-IV aGVHD. (B) Cumulative incidence of cGVHD.
Incidence of graft-vs-host disease. (A) cumulative incidence of grade II-IV aGVHD. (B) Cumulative incidence of cGVHD.
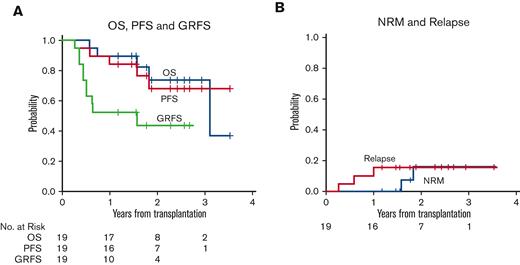
Median follow-up for surviving patients was 25 months (range 13-42 months) following HCT. Three patients (16%) have relapsed during follow-up, at 95, 214, and 363 days following HCT, respectively. Only 1 of these patients was receiving maintenance therapy at time of relapse, with the remaining 2 having previously discontinued enasidenib to pursue treatment for GVHD. Of the 19 patients, 14 remain alive. Five patients (26%) have died to date, at 209, 271, 573, 667, and 1135 days following HCT. Causes of death included disease relapse (n = 3), COVID-19 infection (n = 1), and an unknown cause (n = 1). At 2 years, the cumulative incidence of disease relapse was 16% (95% CI, 3.7-36), and the cumulative incidence of NRM was 16% (95% CI, 2.3-42). Two-year OS was 74% (95% CI, 44-90), and 2-year PFS was 69% (95% CI, 39-86) (Table 3; Figure 3).
Survival, relapse, and non-relapse mortality. (A) OS, PFS, and GRFS. (B) Cumulative incidence of relapse and NRM.
Survival, relapse, and non-relapse mortality. (A) OS, PFS, and GRFS. (B) Cumulative incidence of relapse and NRM.
Among the 3 patients with AML with adverse-risk cytogenetics, 1 demonstrated deletion 7 on karyotypic analysis, and 2 had a complex karyotype. One underwent a RIC Haplo, 1 had a RIC MUD, and 1 underwent MAC MUD transplantation. None of the patients have relapsed, but 1 died of infectious complications following HCT. For the 2 patients alive at 12 months, IDH2 mutations were not detected in the bone marrow at that time.
Baseline demographic, disease, and transplant factors were assessed for any association with PFS and OS, including age, gender, lines of prior therapy, previous enasidenib exposure, concurrent mutations, type of IDH2 mutation, HCT type, and conditioning intensity, but no statistically significant associations with outcome were detected, largely because of the small number of events.
Bone marrow IDH2 mutational burden before transplant was available in 9 patients (Figure 4 and supplemental Figure 1). Among these, 5 had detectable IDH2 mutations, of whom 2 (40%) subsequently relapsed. Among the 4 patients who did not have detectable IDH2 mutations before HCT, 1 (25%) relapsed. In addition, bone marrow mutational burden following HCT, but before initiating enasidenib maintenance, was also available for 14 patients (Figure 4 and supplemental Figure 1). Among these, IDH2 mutations were detectable in 6 patients, of whom 1 (17%) relapsed. Of these patients, 3 no longer had detectable IDH2 mutations at 12 months, and for the remaining 3, IDH2 VAF data were not available at 12 months. Of the 8 patients without detectable mutations before enasidenib initiation, 1 patient (13%) subsequently relapsed. A supplemental Table provides the available IDH2 mutational burden, as well as the concurrent myeloid blast percentage for marrow samples before HCT and before enasidenib treatment, all of which were below 5.0%.
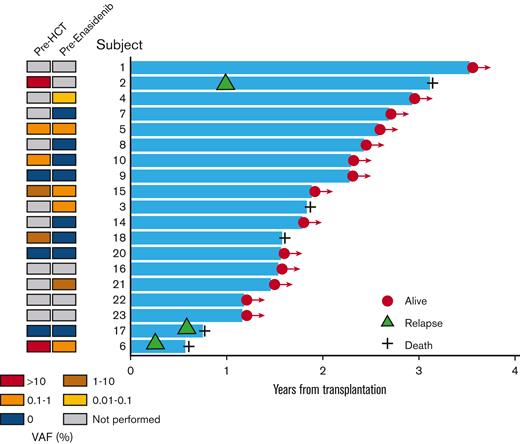
Swim plot of post-HCT outcomes among patients receiving enasidenib maintenance therapy. On the left, a panel shows a heatmap for the status of IDH2 mutational burden before HCT and before enasidenib maintenance. The right panel shows a swim plot for time from HCT to death or last seen alive. Orange triangle indicates relapse, plus sign indicates death.
Swim plot of post-HCT outcomes among patients receiving enasidenib maintenance therapy. On the left, a panel shows a heatmap for the status of IDH2 mutational burden before HCT and before enasidenib maintenance. The right panel shows a swim plot for time from HCT to death or last seen alive. Orange triangle indicates relapse, plus sign indicates death.
Baseline plasma 2-HG levels were available for 16 of 19 patients that underwent treatment. The median 2-HG concentration at study entry for all patients was 55 ng/mL (range <30-349 ng/mL), 51 ng/mL (range <30-88.3 ng/mL) for those with R140 alterations, and 73 ng/mL (<30-349 ng/mL) for those with R172 alterations (P = .32, supplemental Figure 2). For those patients who experienced subsequent relapse during follow-up, all had baseline plasma 2-HG concentration above 90 ng/mL, whereas only 1 out of 13 patients who remained in remission during follow-up had plasma 2-HG concentration above 90 ng/mL. Among those with 2-HG concentrations above 90 ng/mL, all had IDH2 R172K mutations. Although the number of relapsed patients is limited, we explored a possible association between relapse and baseline plasma 2-HG concentration. In a univariable Fine and Gray competing risks model for relapse, plasma 2-HG > 90 ng/mL had subdistribution hazard ratio of 7.1 (95% CI, 0.8-64; P = .08).
Discussion
The clinical development of IDH inhibitors in AML over the last decade has helped transform the therapeutic landscape of AML. Single-arm clinical trials of ivosidenib and enasidenib in patients with R/R AML demonstrated remarkable tolerability and promising efficacy in patients with traditionally poor prognoses.6,8,18 These monotherapy studies led to an approval of both agents for R/R IDH1- and IDH2-mutated AML from the Food and Drug Administration. These agents have subsequently also shown significant clinical activity in combination with hypomethylating agents in newly diagnosed, older patients with AML.23,24
Nevertheless, despite the demonstrated tolerability and efficacy of IDH inhibitors in clinical trials, these orally bioavailable, daily dosed therapeutics have not been studied as maintenance therapy following HCT, a setting in which other targeted therapies have already been studied with substantial therapeutic promise. For example, FLT3 inhibitors have improved outcomes following HCT, with randomized studies of sorafenib following HCT showing improvement in survival and lower rates of relapse.12,13 Whereas prior maintenance approaches, including those with the antibody-drug conjugate gemtuzumab ozogamicin25 and lenalidomide26 did not appear to improve outcomes following HCT, the success of FLT3 inhibitors has reinvigorated interest and promise in this setting. Several post-HCT maintenance studies are ongoing, including those of the BCL2 inhibitor venetoclax (NCT04161885), oral azacitidine (NCT04173533), decitabine/cedazuridine (NCT04980404), and the TP53 activator eprenetapopt (NCT03931291).
Given these considerations, we sought to assess the tolerability and gain an early estimation of efficacy for enasidenib as maintenance therapy following HCT in patients with IDH2-mutation with myeloid malignancies. Enasidenib was well-tolerated at the doses studied, 50 and 100 mg daily. 100 mg daily was determined as the RP2D. Although 8 patients did not complete all protocol-assigned cycles of enasidenib, the majority did complete all 12 cycles. Only 3 patients stopped treatment because of AEs deemed at least possibly related to treatment. Grade 3 or higher toxicities were rare, with the most common attributed toxicities being cytopenias. Transient dose interruptions did occur in 42.1% of patients, and in most, a reduction to 50 mg daily allowed patients to continue and tolerate treatment.
In this study, no cases of grade 3 or higher aGVHD were noted, and only 5 cases of grade 1 or 2 aGVHD occurred following start of enasidenib maintenance, which is reassuring. The cumulative rates of aGVHD and cGVHD on this study approximate that typically seen in transplanted patients, and the cumulative incidence of NRM of 16% also compared favorably to historical data.27,28 One patient experienced severe cGVHD, and 5 required the initiation of steroids for treatment. Although this data appear to suggest that use of enasidenib maintenance does not impact the development of significant aGVHD or cGVHD, true associations can only be defined with results from larger studies of enasidenib following HCT.
Bilirubinemia, a finding commonly seen with enasidenib and associated with a benign course,29 was also uncommon in the current study, with 5 detected cases and 1 reported grade 3 event. Differentiation syndrome, associated with IDH inhibitors, has been studied and detailed in prior clinical trials of these agents.30,31 This cytokine-mediated entity is caused by differentiation of myeloid blasts through targeted IDH inhibition, and its manifestation is clinically heterogeneous often with fevers, pleural infiltrates and effusions, leukocytosis, and rash.30 Although the protocol mandated close monitoring and management guidance for differentiation syndrome, no cases were reported in study patients. This is not surprising, given that all patients that underwent treatment were in assumed remission and with their disease controlled at time of study enrollment, with very low levels of leukemic burden.
In this study, 3 patients have experienced relapse in follow-up. Only 1 relapsed while on enasidenib, with the remaining 2 relapsing after ending maintenance early to pursue therapy for GVHD. Among 19 patients treated with enasidenib following HCT, we found an estimated PFS and OS at 2 years of 69% and 74%, respectively. In recent years, a few studies have investigated the post-HCT outcomes of patients with IDH1/2 mutation with AML. In general, outcomes for IDH-mutated cohorts seem to approximate historical outcomes of the general AML population. One smaller study assessed 23 patients with IDH mutation with AML and reported 1-year OS and relapse rates of 68% and 29%. Of note, these outcomes were not further defined by IDH1 and IDH2 subtypes, and the median follow-up was brief, at 7.8 months.28 A larger, multicenter retrospective study of transplanted patients with IDH mutation, with a median follow-up of 25.2 months, reported 2-year PFS and OS of 58% and 68%, respectively for the IDH2-mutated cohort. The 2-year cumulative incidence of relapse and NRM was 19.6% and 25.2%, respectively.27 The number of patients in our phase 1 study is limited, but the estimated 2-year PFS and OS data among patients treated with enasidenib, and the cumulative incidence of relapse of 16%, does compare favorably to this historical experience. However, 2 of our patients with IDH2 mutation had MDS with multiple prior lines of treatment, and among the 17 patients with AML, a larger than expected proportion for an IDH2-mutated cohort (9 patients, 52.9%) had AML arising from an antecedent myeloid neoplasm. Therefore, studies in a larger population of patients with IDH2-mutation are necessary to demonstrate an advantage for enasidenib maintenance following HCT.
Baseline 2-HG levels were suppressed for patients subsequently treated with maintenance, with a median concentration of 55 ng/mL. This is in line with published data on 2-HG concentrations noted among healthy individuals32 and those responding on trials.6,8 Although the number of patients that relapsed was small, there was a trend toward an association between baseline plasma 2-HG and subsequent relapse during follow-up.
Assessment of IDH2 clonal burden, as a measure of MRD, were assessed before HCT and before start of maintenance in a subset of our patients. In general, albeit truly limited by the number of patients that were assessed, a detectable IDH2 mutation was associated with a numerically higher rate of subsequent relapse and death. For 3 patients with both datapoints available, an IDH2 mutation detected at time of enasidenib initiation was no longer detected at 12 months following HCT. Previous studies have demonstrated that disease detection at the time of transplant and before maintenance therapy is associated with worse outcomes in AML.33-37 However, larger randomized studies are needed to fully assess the prognostic and predictive role of IDH mutation–based MRD in patients receiving post-HCT maintenance with IDH inhibitors. These efforts should be incorporated in any future study of this and other maintenance therapies in AML.
Limitations of this study included its small sample size, with this phase 1 clinical trial of enasidenib maintenance therapy enrolling 22 patients and treating 19 with IDH2 mutations. Accurate estimations of activity, the role of MRD, and long-term efficacy require larger studies. In addition, although this was a multicenter effort, only 3 large academic sites accrued participants, and the effects of demographics, regional treatment and transplant patterns, and variations in patient selection for clinical trials may not be representative of larger populations. Our study population was also somewhat heterogeneous and included patients with both MDS and AML, along with patients with both newly diagnosed and R/R disease, although 16 of 19 patients that underwent treatment did have newly diagnosed AML before HCT. Assessment of outcomes, therefore, has to be considered within this important context.
In summary, to the best of our knowledge, this is the first attempt to assess the tolerability and activity of IDH2 inhibition following HCT for myeloid malignancies. Enasidenib was well-tolerated, and we established a RP2D of 100 mg daily. Most of the patients that underwent treatment completed 12 months of planned maintenance therapy following HCT. The incidence of relapse was low and the estimated 2-year PFS and OS with enasidenib maintenance in this small phase 1 study was promising. Nevertheless, larger randomized studies are needed to truly assess the potential of this drug as maintenance therapy following HCT and to improve outcomes for our patients with IDH2 mutated myeloid malignancies.
Acknowledgment
Celgene Pharmaceuticals (now Bristol Myers Squibb) kindly provided funding and supply of enasidenib for the conduct of this study. Agios Pharmaceuticals (now Servier) kindly provided funding for correlative IDH2 mutational analysis.
Authorship
Contribution: A.T.F. developed the study design, performed data collection, supervised overall conduct of the study, and wrote the manuscript; H.T.K. and S.L. performed biostatistical analysis of data; R.J.S., M.J.L., A.S.M., Z.D., A.E.-J, S.L.M., A.M.B., R.N., V.T.H., and Y.-B.C. helped with study design and development, conduct of the study, and edited the final version of the manuscript; A.S.K. performed correlative laboratory analysis; L.W.K. and D.K. helped with multicenter conduct of study, study accrual, and helped edit the manuscript; and A.J.S.B., L.H.P., J.L.W., J.B., and E.B. helped with sample collection, study evaluations and accrual, and edited the final version of the manuscript.
Conflicts-of-interest disclosure: A.T.F. has consulted for Agios, Celgene, Bristol Myers Squibb, Servier Pharmaceuticals, Forma Pharmaceuticals, Astellas, Amgen, Takeda, Immunogen, AbbVie, Mablytics, Ipsen, Genentech, EnClear, Orum, and Novartis. Z.D. has consulted for Syndax Pharmaceuticals, Kadmon, Omeros, Incyte, and MorphoSys. A.E.-J. has consulted for Novartis, GSK, and Incyte. A.M.B. has consulted for Acceleron, Agios, BMS/Celgene, CTI BioPharma, Gilead, Keros Therapeutics, Novartis, Taiho, and Takeda. Y.-B.C. has consulted for Incyte, Magenta, Moderna, Novartis, Gamida Cell, T-Scan, Actinium, and Equilium. The remaining authors declare no competing financial interests.
Correspondence: Amir T. Fathi, Massachusetts General Hospital, Harvard Medical School, Zero Emerson Place, Suite 118, Boston, MA 02114; e-mail: afathi@partners.org.
References
Author notes
Data, including datasets and protocol, are available on request from the corresponding author, Amir T. Fathi (afathi@partners.org). We agree to freely share publication-related data.
The full-text version of this article contains a data supplement.