Key Points
In a large older cohort, MGUS and CH associate with different clinical and laboratory variables.
MGUS and CH do not cooccur frequently and instead follow two unrelated clonal trajectories within the bone marrow.
Abstract
Monoclonal gammopathy of undetermined significance (MGUS) and clonal hematopoiesis (CH) are 2 preclinical clonal expansions of hematopoietic cells whose prevalence rises with age, reaching almost 10% in people of aged 70 years and older. The increased risk of myeloid malignancies in patients with myeloma is well defined, and the study of the association between CH and MGUS could help explain this phenomenon. Here, we analyzed a fully clinically annotated dataset of 777 older subjects (median age, 91 years) previously screened for prevalence of CH. The prevalence of MGUS and CH was 9.6% and 17.3%, respectively. We detected CH in 9.7% of the patients with MGUS and MGUS in 5.5% of the patients with CH. We did not find a significant correlation between the presence of MGUS and CH. Furthermore, the 2 conditions showed a differential association with clinical and laboratory covariates, suggesting that MGUS and CH may represent age-associated unrelated clonal drifts of hematopoietic cells. Confirmatory studies are needed to assess the relevance of CH in plasma cell disorders. This trial was registered at www.clinicaltrials.gov as #NCT03907553.
Introduction
Cancer is defined by a multistep acquisition of driver clonal abnormalities resulting in expansion of malignant cell populations. The identification of preclinical cancer stages has for long been restricted to cases with phenotypic manifestations (eg, production of a monoclonal antibody in the case of monoclonal gammopathy of undetermined significance [MGUS]).1 Novel genomic techniques have conversely expanded the knowledge of preclinical cancer stages in several solid and hematological tumors.2,3 Aside from MGUS, sustained by bone marrow clonal plasma cells (PC), a preclinical clonal expansion was recently described in hematopoietic stem cells by genomic techniques in a condition called clonal hematopoiesis (CH).4 This new clonal entity is defined by the presence of somatic gene mutations in white blood cells of people without any sign of hematological malignancy.4-8 Importantly, a subset of CH cases is referred to as clonal hematopoiesis of indeterminate potential (CHIP) to reflect the presence of driver gene mutations found in leukemia and a higher propensity toward transformation.
Both CH and MGUS are defined as two aging-related entities because their prevalence increases in the elderly population reaching more than 10% for CH and 6% for MGUS over the age of 70 years.4-6,9-11 Importantly, patients with CH or MGUS are asymptomatic but at risk for evolution into acute myeloid leukemia or multiple myeloma (MM), respectively.4,5,7,10 Recent data highlight shared clinical trajectories of these conditions. The presence of CH has been described as an adverse prognostic factor for MM relapse after treatment.12 Furthermore, patients with MM are at increased risk of developing myeloid malignancies, both in sequence or synchronously.13,14 Of note, this risk can be explained by the use of cytotoxic therapy in patients with MM undergoing autologous stem cell transplantation, but cannot be explained in asymptomatic patients, raising the question as to whether there is a shared clonal phylogeny between these myeloid and PC malignancies.13 However, data from a large study on myeloid and lymphoid clonal hematopoiesis and their additive risk of development of myeloid and lymphoid malignancies have been published.15 Interestingly, the presence of CH was not associated with an increased risk of development of MM in this recent paper.15
We hypothesized that the study of the association and dynamics of the 2 precursor conditions CH and MGUS may help shed some light on the possible hierarchical clonal interrelationships between myeloid and PC malignancies. To this end, we analyzed a unique older population dataset, annotated for both MGUS and CH, and their association with other clinical and laboratory variables.
Methods
Study cohort
In this study, we analyzed clinical and genomic data derived from 777 subjects enrolled in the Monzino 80-plus cohort who was previously used to study dementia in a large population-based study16 and for subsequent genomic analysis on CH prevalence.17 The study population and sequencing procedures were already described.17 For the purpose of our study, we called and filtered variants as described in supplemental Methods. We applied a minimum cutoff of 0.02 for variant allele frequency. Written informed consent was obtained before blood sampling.
Statistical analysis
Continuous variables were reported by median and range. Prevalence of categorical variables was expressed by absolute numbers and relative frequency. The association between CH and MGUS was evaluated by the Fisher exact test. Wilcoxon test was used to define possible differences in the distribution of continuous variables between patients carrying MGUS or not. Subsequently, using a linear regression model, we assessed possible correlations between age as an independent continuous variable and other predetermined dependent variables such as hemoglobin, albumin, mean corpuscular volume (MCV), and γ-globulin concentration. Finally, to better define a possible correlation or anticorrelation of the previously mentioned variables with MGUS and CH, we implemented uni- and multivariate logistic regression models using MGUS or CH as an independent variable. All analyses were performed in R using the “stats” R package version 4.0.4. P values were corrected by Bonferroni-Hochberg procedure.
Results and discussion
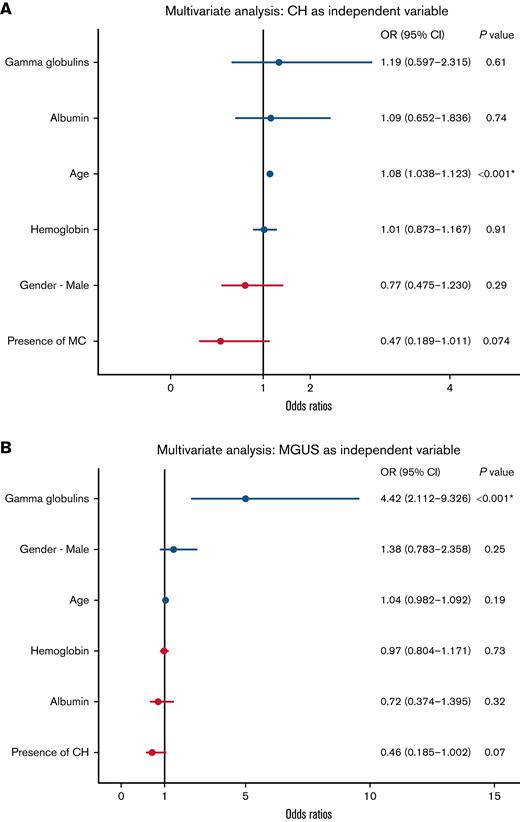
Good-quality sequence data from unsorted peripheral blood samples were available for 43 genes in 732/777 patients.17 Serum protein electrophoresis data were available for the entire cohort. The median age of the cohort was aged 91 years (range, 81-104), significantly higher than other previously published MGUS studies.11 Other clinical and laboratory features of the cohort are shown in Table 1. We detected a prevalence of MGUS and CH of 9.6% (75/777) and 17.3% (127/732), respectively. The complete list of identified mutations and the distribution of their allelic frequencies are reported in supplemental Table 1 and supplemental Figure 1, respectively. Mutations were filtered for germline and artifacts based on public databases but also using sequencing data generated ad-hoc from a panel of 32 healthy subjects of a maximum of 30 years of age (see supplemental Methods for details). As expected, people harboring MGUS showed higher levels of γ-globulins (1.18 g/dL vs 1.05 g/dL, Wilcoxon test, P value = .006439) (supplemental Figure 2A). Surprisingly, no significant association was found between MGUS and age, nor with hemoglobin concentration, albumin levels, or MCV (supplemental Figure 2B-E). We then assessed the impact of age as a continuous variable on specific laboratory features. Of note, albumin and hemoglobin concentration significantly decreased with age (supplemental Figure 3A-B; P values, 8.2e-12 and 8.6e-15, respectively). On the other hand, no significant correlation was found between age and γ-globulin concentration or MCV (supplemental Figure 3C-D). A significant anticorrelation between albumin and γ-globulin levels was also confirmed (P = .0022; supplemental Figure 3E). Then, we focused on the possible association between MGUS and CH: 7 patients carried both CH and MGUS. The prevalence of CH in patients with MGUS was 9.7% (7/72) and the prevalence of MGUS in patients with CH was 5.5% (7/127). A Fisher exact test showed no association between the 2 clonal events but rather a nonsignificant trend toward anticorrelation (P = .073). To further explore this result, we implemented both uni- and multivariate logistic models using presence/absence of MGUS or CH as independent dichotomic variables. By univariate analysis, high γ-globulins and low albumin were associated with MGUS (Figure 1A; supplemental Table 2A). In multivariate analysis, the presence of MGUS was only significantly associated with higher levels of γ-globulins (estimation: 1.48656; P = 7.74e-05) but not with age, sex, hemoglobin, or albumin concentration (Figure 1A; supplemental Table 2A). This analysis confirmed a trend of anticorrelation between MGUS and CH (estimation: -0.76713; P = .0707) (supplemental Table 2A,B). On the other hand, CH as an independent variable showed a significant correlation with age (estimation: 0.076308; P = .000158) (Figure 1B; supplemental Table 2B). As for the type of mutated genes, CH showed an expected pattern. Because of low numbers, we did not find any significant difference in mutational patterns between CH patients with or without MGUS (supplemental Figure 4).
Forest plots of multivariate analyses to determine the possible association between MGUS and CH. (A) Forest plot using CH as independent variable. (B) Forest plot using MGUS as independent variable. CI, confidence interval; OR, odds ratio.
Forest plots of multivariate analyses to determine the possible association between MGUS and CH. (A) Forest plot using CH as independent variable. (B) Forest plot using MGUS as independent variable. CI, confidence interval; OR, odds ratio.
Because CHIP defines, among CH cases, those bearing the known leukemia driver gene mutations and the highest propensity to transformation, we also asked whether the subset of our patients bearing CHIP showed a similar pattern. To this end, we restricted our analysis to cases showing clonality in accordance with the recently published list of gene mutations more frequently related to myeloid neoplasms (M-CHIP) as done previously.15 Again, MGUS and M-CHIP showed association with different clinical and laboratory variables and no significant association with each other (supplemental Table 3).
CH is associated with reduced survival, partly, but not predominantly, from the risk of evolution to overt myeloid malignancies.5,6,12,18 Intriguingly, CH is a risk factor for cerebro-cardiovascular events5,6 and is associated with autoimmune diseases in patients undergoing hip replacement for osteoarthritis.18 Studies have reported an increased risk of MDS/acute myeloid leukemia development in MGUS13 and in patients with MM undergoing lenalidomide maintenance.19-21 In this latter case, CH could represent the grounds on which the pharmacological intervention induces evolution to a myeloid neoplasm maintaining, at the same time, the plasma cell dyscrasia under control. In asymptomatic cases, though, the increased risk may suggest a shared origin of the 2 malignancies. On the other hand, it has been recently demonstrated that CH represents an independent risk factor for progression in patients with asymptomatic Waldenstrom macroglobulinemia.22 However, the association of CH with other preclinical bone marrow clonal disorders has not been studied in a general patient population, and so the question is still open as to whether there is a hierarchy of clonal conditions and a causal relation between them. Here, interrogating a well-annotated dataset of 777 older patients, we found no association between CH and MGUS, but on the contrary a tendency toward their mutual exclusivity. Furthermore, the two conditions tended to have different clinical and laboratory covariates, suggesting that they develop along independent biological trajectories. Indeed, our results could be biased by the peculiar features of our cohort, and we may be reporting data on a selected population that may limit their wider application. In fact, our cohort is significantly older than any other study in MGUS and skewed toward the female sex, characterized by a lower prevalence of MGUS and a longer life expectancy. Additionally, we may have selected a somehow favorable subset of patients, whereas subjects harboring MGUS and CH for a long time could have already died from disease progression before sampling. However, the lack of association between CHIP and subsequent development of MGUS or MM was recently described by Niroula et al,15 and our findings here may well be explained by the same biological phenomenon. Moreover, our data in asymptomatic people are also in line with a recent report on the lack of a common progenitor between myelodysplastic and myeloma clones in rare patients harboring both diseases,23 and may point at different interactions between these 2 clonal conditions (ie, through the microenvironment). In conclusion, our study complements existing evidence in overt cancer conditions and sheds new light on an increasingly overrepresented setting of patients, usually understudied and on which biological data are generally scanty.
Acknowledgments
The Monzino 80-plus Study is supported by a research grant from Italo Monzino Foundation, Milan, Italy. This work was supported by the European Research Council under the European Union’s Horizon 2020 research and innovation program (817997, to N.B.); Associazione Italiana Ricerca sul Cancro (IG16722, IG10136, and IG24365 to A.N., IG25739 to N.B., and IG22053 to M.G.D.P.); Fondazione Umberto Veronesi (Umberto Veronesi Foundation) to M.C.D.V.; PRIN 2017 (Ministry of University & Research, Italy – Project 2017WXR7ZT to M.G.D.P.); Ricerca Finalizzata 2016 (Italian Ministry of Health, Italy – Project RF2016-02364918 to M.G.D.P. and U.L.); and Cariplo Foundation (Milan Italy - Project # 2016-0860 to M.G.D.P. and U.L.).
Authorship
Contribution: M.G.D.P. and N.B. designed the study; A.A.G., U.L., S.M., E.R., and M.T. provided samples and clinical data; M.C.D.V., M.L., A. Marella, and A. Matera analyzed data; M.C.D.V., M.L., A. Matera, E.S., L.P., A.P., L.B., A.N., and N.B. interpreted data; M.C.D.V., M.L., and N.B. wrote the paper; E.T. performed next-generation sequencing experiments; and all authors approved the paper for submission.
Conflict-of-interest disclosure: N.B. received honoraria from Celgene, Amgen and Janssen. The other authors declare no competing financial interests.
The current affiliation for A.N. is Scientific Directorate, Azienda USL-IRCCS, Reggio Emilia, Italy.
Correspondence: Niccolò Bolli, Hematology Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico - Department of Oncology and Hemato-Oncology, University of Milan, via Francesco Sforza, 35, 20122, Milan, Italy; e-mail: niccolo.bolli@unimi.it.
References
Author notes
Data are found under accession number PRJNA736552. Requests for data sharing may be submitted to Niccolò Bolli (niccolo.bolli@unimi.it.)
The full-text version of this article contains a data supplement.