Key Points
Patients with CLL progressing on zanubrutinib have an enrichment of the BTK Leu528Trp compared with those who progress on ibrutinib.
Cross-resistance may be observed in patients who progress while on zanubrutinib with BTK Leu528Trp mutations and subsequent pirtobrutinib.
Abstract
The covalent Bruton’s tyrosine kinase inhibitors (BTKis) are highly effective for the treatment of chronic lymphocytic leukemia (CLL). The dominant resistance mechanism observed with the BTKi ibrutinib is the development of BTK Cys481 codon mutations. Whether a similar resistance mutation profile exists for the newer-generation, more selective BTKi zanubrutinib is unknown. In samples referred for diagnostic next-generation sequencing in patients with progressive CLL, we observed an enrichment in the kinase-dead BTK Leu528Trp mutation in patients treated with zanubrutinib compared with ibrutinib (54%; 7 of 13 vs 4%; 1 of 24, P = .001). We describe 2 patients with BTK Leu528Trp mutations who showed clinical cross-resistance and progressive enrichment of the BTK Leu528Trp mutation over time when treated with the noncovalent BTKi pirtobrutinib. Both patients subsequently responded to venetoclax-based treatment. In summary, we have identified an enrichment of the BTK Leu528Trp mutation arising in patients treated with zanubrutinib that may impart cross-resistance to the noncovalent inhibitor pirtobrutinib and therefore may have implications for sequencing of these treatments in CLL.
Introduction
Covalent Bruton’s tyrosine kinase inhibitors (BTKis) are a highly effective drug class for the treatment of chronic lymphocytic leukemia (CLL).1-3 Ibrutinib was the first covalent BTK inhibitor to be clinically evaluated, followed by multiple “second-generation” covalent BTK inhibitors that are more selective for BTK and have fewer off-target adverse effects.4,5 Zanubrutinib is a highly selective second-generation covalent BTKi with demonstrated efficacy in both frontline and relapsed/refractory CLL.6
Most of our understanding of clinical resistance to covalent BTKis comes from patients treated with ibrutinib. Both ibrutinib and second-generation inhibitors bind covalently to the Cys481 residue of BTK and block its activity.7 The predominant identifiable mechanism of resistance in patients treated with ibrutinib and acalabrutinib is the development of mutations in BTK, most commonly Cys481Ser,8,9 resulting in reduction of affinity of these BTKis for their target.8 Noncovalent BTK inhibitors such as pirtobrutinib have since been developed that have shown high efficacy in relapsed and refractory CLL and importantly in patients with disease carrying Cys481 mutations.10
In contrast to ibrutinib and acalabrutinib, the spectrum of resistance mutations to the second-generation covalent BTKi zanubrutinib is less well described. We have reported that the BTK Leu528Trp mutation was potentially enriched in patients with disease that progressed while they received zanubrutinib compared with ibrutinib.11 More recently, the BTK Leu528Trp mutation has also been observed in multiple patients with disease progression during pirtobrutinib treatment.12 Given the potential for cross-resistance between zanubrutinib and pirtobrutinib, we sought to further characterize the landscape of BTK resistance mutations in patients with disease that progresses during zanubrutinib treatment, in the context of subsequent noncovalent BTK inhibitor therapy.
Methods
Mutation data from consecutive samples referred for diagnostic targeted DNA-sequencing for investigation of hematological malignancy at the Peter MacCallum Cancer Centre from 2017 to February 2022 were reviewed to identify specimens with mutations in BTK among patients with CLL. Targeted next-generation sequencing was performed with a combination of targeted amplicon sequencing with a Fluidigm access array and unique molecular index–based, error-corrected sequencing for a panel of genes (including BTK [NM_000061.2] exons 11, 15-16) recurrently mutated in hematological malignancy, as previously described.13,14 The study was approved by the Peter MacCallum Cancer Centre Human Research Ethics Committee and performed according to the Declaration of Helsinki.
Results and discussion
Sixty-three variants from 37 patients were identified in BTK among samples from patients with CLL treated with either ibrutinib (n = 24) or zanubrutinib (n = 13) (supplemental Tables 1 and 2). No patients had previous acalabrutinib treatment or prior BTKi exposure documented. When analyzed with regard to the specific BTKi received, we observed an enrichment of BTK Leu528Trp mutations occurring in patients treated with zanubrutinib when compared with ibrutinib (54%; 7 of 13 vs 4% 1 of 24; P = .001, Fisher’s exact test; Table 1). The rarity of Leu528Trp mutations in ibrutinib-treated patients in our cohort is consistent with the literature from ibrutinib-resistant cohorts to date.15-18
An analysis of disease progression samples from patients treated with pirtobrutinib in the BRUIN trial has described the emergence of multiple non-Cys481 BTK mutations (including the BTK Leu528Trp) that have been hypothesized to contribute to secondary pirtobrutinib resistance12 in 7 of 9 (78%) patients. Given the emergence of BTK Leu528Trp mutations in patients with disease progression during treatment with either zanubrutinib or pirtobrutinib, we sought to understand the extent and implications of any potential genomic cross-resistance between these 2 agents.
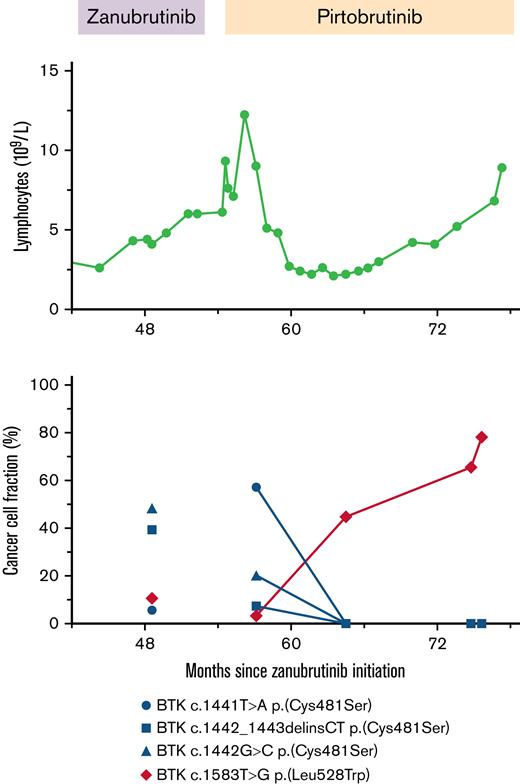
Two patients were identified from our institution who had received pirtobrutinib after disease progression during treatment with zanubrutinib. The first was a 58-year-old man diagnosed with CLL 15 years prior. He was initially treated with FCR (fludarabine+cyclophosphamide+rituximab) and achieved a measurable disease–negative (MRD-negative) complete remission (CR). At disease progression 4 years later, he received zanubrutinib and achieved a second CR. His disease subsequently progressed 4 years later and molecular testing at this stage demonstrated 3 dominant BTK Cys481Ser mutations, as well as a BTK Leu528Trp mutation, with a cancer cell fraction (CCF) of 2.2%. Previous single-cell sequencing performed on samples from this patient had shown the BTK mutations to be present in different clones.19 He was treated in a clinical trial with pirtobrutinib (registered on https://www.clinicaltrials.gov as #NCT03740529) and achieved a partial response. On pirtobrutinib, a decrease in CCF of his Cys481Ser-mutated clones was observed, with a concomitant progressive enrichment in his disease compartment of the BTK Leu528Trp clone without development of further BTK or PLCG2 mutations (Figure 1). He met the formal definition of disease progression 23 months after commencing pirtobrutinib. He was subsequently treated with venetoclax and rituximab with resolution of peripheral blood lymphocytosis at 1 month of therapy.
Longitudinal disease and mutation assessment in a patient with disease progressing on zanubrutinib and pirtobrutinib. Peripheral blood lymphocyte count over time during transition from zanubrutinib to pirtobrutinib therapy (top). CCF of detectable BTK mutations over the same period. Blue symbols indicate mutations corresponding to the Cys481Ser amino acid change; red symbols indicate the mutation corresponding to the kinase-dead Leu528Trp mutation (bottom). Changes in CCF during pirtobrutinib therapy are annotated with a connecting line.
Longitudinal disease and mutation assessment in a patient with disease progressing on zanubrutinib and pirtobrutinib. Peripheral blood lymphocyte count over time during transition from zanubrutinib to pirtobrutinib therapy (top). CCF of detectable BTK mutations over the same period. Blue symbols indicate mutations corresponding to the Cys481Ser amino acid change; red symbols indicate the mutation corresponding to the kinase-dead Leu528Trp mutation (bottom). Changes in CCF during pirtobrutinib therapy are annotated with a connecting line.
The second patient was a 63-year-old woman diagnosed with CLL 15 years previously. She was initially treated with FCR and achieved a CR, followed by disease progression 7 years later. She was then treated with zanubrutinib and achieved a partial response, with disease progression after 5 years of therapy. At progression, molecular testing showed a completely clonal (CCF, 100%) BTK Leu528Trp mutation. She began pirtobrutinib (NCT03740529) and had stable disease as her best response, before demonstrating nodal disease progression after 6 months of therapy. A bone marrow aspirate sample taken at the time of nodal progression again showed an ongoing clonal BTK Leu528Trp mutation (CCF, 100%) without any new BTK or PLCG2 mutations observed. She was subsequently treated with venetoclax and rituximab and achieved an MRD-negative CR.
The enrichment of BTK Leu528Trp mutations at disease progression in patients treated with zanubrutinib is of potential significant clinical relevance, as illustrated by the 2 patients described. Both patients received pirtobrutinib therapy, with BTK Leu528Trp mutations after preceding zanubrutinib therapy, with 1 patient showing progressive enrichment of an initially less-dominant BTK Leu528Trp clone over time and the other patient (with a dominant initial BTK Leu528Trp clone) showing primary resistance to therapy. Samples from these patients were not included in the recently published work on pirtobrutinib resistance from the BRUIN trial.12 Preexisting BTK Leu528Trp mutations and their potential cross-resistance may partially explain the suboptimal response to pirtobrutinib in these 2 cases. Importantly, both patients showed evidence of clinical response to venetoclax/rituximab (including 1 patient who achieved an MRD-negative response) despite resistance to pirtobrutinib therapy.
The BTK Leu528Trp mutation prevents the binding of multiple BTKis including ibrutinib, zanubrutinib, and pirtobrutinib.11,12,20 However, it also prevents binding of adenosine triphosphate, resulting in a protein that lacks kinase activity (“kinase-dead”). Despite the lack of kinase activity, phosphorylation of downstream phospholipase Cγ2 has been observed in cells harboring these mutations.21,22 Although the molecular basis of bypassing phenomena is unknown for Leu528Trp, biochemical studies of other kinase-dead mutations (Cys481Phe and Cys481Tyr) have implicated other intracellular kinases, including HCK, as being involved in reestablishing BTK signaling.22 An important unresolved question is what drives the preferential development of BTK Leu528Trp mutations. As they are observed in both the covalent and noncovalent BTKi setting, other factors, such as the selectivity and kinome profile of the various agents and any potential off-target effects of nonselective BTKis on bypassing mechanisms, warrant further exploration.
In summary, we observed enrichment of the kinase-dead BTK Leu528Trp mutation in our cohort of patients with CLL that progressed during zanubrutinib treatment when compared with ibrutinib, which has potential implications for choice of BTKi and subsequent therapies where this mutation is suspected to confer resistance, such as pirtobrutinib. Further interrogation of larger cohorts is needed to clarify the possibility of cross-resistance and the ramifications for sequencing of BTKis. Detailed studies on whether Leu528Trp and other kinase-dead BTK mutations are preferentially selected for patients receiving other highly selective BTKis are warranted.
Acknowledgments
The authors thank Clarissa Wilson for coordination of the patient samples.
This study was supported by the Wilson Centre for Lymphoma Genomics (P.B. and E.R.T.), the Snowdome Foundation (P.B. and E.R.T.), and the National Health Medical Research Council (grants 1174902 and 2011139) (A.R.).
Authorship
Contribution: P.B. designed the study, treated patients, and analyzed the molecular data; T.E.L., E.R.T, and I.S.T. analyzed the molecular data and wrote the paper; R.B., C.Y.C., K.L.L., S.M.H., C.P.S.T., A.R., and J.F.S., contributed samples and treated patients; C.S.T. treated patients and supervised the study; and all authors contributed to writing the manuscript and approved the final version.
Conflict-of-interest disclosure: T.E.L. is a past employee and A.R. is an employee of the Walter and Eliza Hall Institute of Medical Research, which receives milestone and royalty payments related to venetoclax. Both are recipients of a share in royalty payments paid to the Walter and Eliza Hall Institute of Medical Research. T.E.L. has received honoraria from AbbVie. A.R. has received research funding from Janssen and AbbVie. P.B. reports receiving consulting and advisory fees and honoraria from Adaptive Biotechnologies, AstraZeneca, and Servier. I.S.T. reports receiving consulting fees and honoraria from Pfizer, Servicer, and Amgen. C.S.T. reports receiving honoraria from AbbVie, BeiGene, Janssen, Novartis, and Roche and research support from AbbVie and Janssen. J.F.S. has been a consultant to Celgene and F. Hoffmann-La Roche Ltd; has received research funding from AbbVie, Celgene, Janssen, and F. Hoffmann-La Roche Ltd; serves on speakers bureaus for AbbVie, Celgene, and F. Hoffmann-La Roche Ltd; has membership on the board of directors or advisory committees for and has received honoraria from AbbVie, AstraZeneca, BMS, Gilead, Janssen, Mei Pharma, Morphosys, F. Hoffmann-La Roche Ltd, Sunesis, and Takeda. C.Y.C. reports receiving consulting and advisory fees and honoraria from Roche, Janssen, MSD, Gilead, AstraZeneca, Eli Lilly, TG Therapeutics, Beigene, Novartis, and BMS and research funding from BMS, Roche, AbbVie, and MSD. S.M.H. has received travel grants from AbbVie and Novartis and honoraria from Roche and Gilead. K.L.L. reports membership on a board or advisory committee/honoraria/patents and royalties and consultancy for AstraZeneca, IQVIA, Janssen, Loxo/Lilly, Novartis, and Roche. The remaining authors declare no competing financial interests.
Correspondence: Piers Blombery, Peter MacCallum Cancer Centre, Grattan St., Melbourne, VIC 3000, Australia; e-mail: piers.blombery@petermac.org.
References
Author notes
Contact the corresponding author for original data (piers.blombery@petermac.org).
The full-text version of this article contains a data supplement.
final version published online 13 October 2022