TO THE EDITOR:
Oncologists cite limited time and resources in busy practices as major barriers to implementing geriatric and frailty assessments,1 which are now recommended for all older adults with cancer undergoing systemic treatment.2 The COVID-19 pandemic has further challenged implementation by reducing the number of in-person clinic visits during which frailty assessments might occur. To overcome these barriers to conducting geriatric and frailty assessments, clinicians and researchers have developed virtual assessments for use in videoconference or telephone visits.3 -6 However, analyses regarding the feasibility of such assessments in older adults with blood cancers are sparse. Moreover, most virtual assessments consist of patient-reported measures, without objective performance measures such as standardized tests of mobility or cognition. We and others have shown that these measures are important predictors of outcome in this patient population.7 -10 Accordingly, we developed and tested a virtual frailty assessment for older adults with hematologic malignancies that incorporates both patient-reported and objective performance measures.
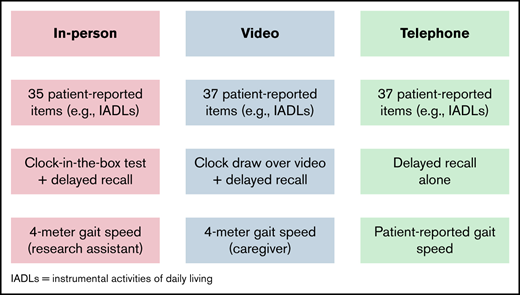
Detailed methods and the analysis plan are provided in the data supplement. Briefly, this was an observational study of transplantation-ineligible patients with blood cancers who enrolled in the Older Adult Hematologic Malignancies Program after presenting for their initial consult at Dana-Farber Cancer Institute (DFCI; Boston, MA).7,8,11,12 We included separate cohorts of patients who were assessed in person (age ≥75 years) and virtually (age ≥73 years). For our in-person cohort, those who consented to participate in the study underwent an in-person screening geriatric assessment administered by a research assistant on the same day as his/her initial hematologic oncology consultation, as described previously.7 The screening geriatric assessment included patient-reported and objective measures, spanning the domains of comorbidity, functional status (eg, instrumental activities of daily living), physical performance (eg, gait speed), and cognition (eg, delayed recall and the clock-in-the box test13 ). All in-person measures collected are included in supplemental Table 1, and detailed scoring of each measure is included in supplemental Table 2. We enrolled patients from February 2015 to March 2020, after which observational studies at DFCI were placed on hold because of the COVID-19 pandemic; partial in-person enrollment resumed in June 2021. We included patients enrolled in person through March 2022, with the exception of a 4-week pause in in-person enrollment in January 2022 because of a rise in coronavirus cases.
From the results of the screening geriatric assessment, we derived frailty status using both phenotypic and deficit-accumulation approaches, 2 of the most widely studied approaches to measuring frailty in aging research (protocol in supplemental Table 2 provides additional details regarding these approaches and their cutoff values that classified severity of frailty).14,15 For both in-person and virtual assessments, we classified patients as robust, prefrail, or frail based on the phenotypic approach, the deficit-accumulation approach, and overall by the more severe classification between both approaches.
To virtually adapt our screening geriatric assessment (supplemental Table 1), patient-reported items were readily converted to administration over video- or teleconference by a research assistant. The data supplement describes our adaptation of objective performance measures. We began enrolling patients for virtual frailty assessments in November 2020 and included patients enrolled through March 2022.
During the period of enrollment for virtual assessments, 254 eligible patients were contacted for recruitment into our study, and 185 (72.8%) consented to enroll (supplemental Figure 1). Of those enrolled, 150 (81.1%) completed the virtual assessment. No falls or other safety events occurred during the virtual assessments. During the period of enrollment for in-person assessments, 1017 patients were approached, of whom 876 (86.1%) enrolled and completed assessments. Table 1 presents the baseline characteristics of the population, restricted to those age ≥75 years.
Among patients age ≥75 years, we did not find differences in the distribution of age, sex, disease type, or self-reported Eastern Cooperative Oncology Group (ECOG) performance score (PS) between in-person and virtual assessments (Table 1). Across frailty measures (overall frailty status, frailty phenotype, and frailty by deficit accumulation), we observed a slightly lower proportion of prefrail and frail patients who completed virtual assessments compared with those who completed in-person assessments. In univariable ordinal regression models (Table 2), virtual assessments trended toward lower odds of classifying patients as overall frail (odds ratio [OR], 0.76; 95% confidence interval [CI], 0.52-1.11), as frail by the phenotypic approach (OR, 0.66; 95% CI, 0.45-0.98), and as frail by the deficit accumulation approach (OR, 0.75; 95% CI, 0.51-1.11). These trends weakened in multivariable ordinal regression models adjusting for age, sex, disease type, and self-reported ECOG PS.
Our findings suggest that virtual frailty assessments entailing both patient-reported and objective performance measures are safe and feasible but may be associated with less severe frailty classification when compared with in-person assessments. Given that this association weakened after adjustment for any differences between assessment type with respect to age, sex, disease type, and ECOG PS, the differences in frailty classification may be better explained by the differences between the populations completing each assessment rather than by differences inherent in the assessments themselves. A more ideal design to compare differences between assessments would have been to measure both in the same individuals from 1 cohort; however, this design was not possible, because many of our virtual assessments took place during surges in the pandemic, when in-person assessments were high risk. Even if our virtual frailty assessment is less sensitive at detecting frailty, the degree of reduced sensitivity is small and must be balanced against the increased burden and risk of in-person assessments. In our example, our virtual frailty assessment allowed our research and clinical program for older adults with blood cancers to continue through several waves of the pandemic and could allow for decentralization of assessments beyond the pandemic to potentially reach more older adults with blood cancers.
We bring specific data from patients with hematologic malignancies into the expanding literature on virtual assessment and care in older adults from other populations.16 -20 The high percentage of our patients who completed virtual assessments is encouraging, especially given that other studies have identified lower uptake of telehealth among older adults compared with younger populations.18,19 Further education regarding the purpose and benefits of frailty assessment could increase our enrollment rate, which was lower than our in-person enrollment rate. This lower rate may in part be due to the fact that our virtual frailty assessments required an additional appointment in the days after initial contact and consent, whereas our in-person assessments occurred at the same time we approached patients for consent while they were waiting for their appointment at DFCI.
Our adaptation of gait speed and cognitive assessment to a virtual format is of particular interest to clinical and research programs focused on older adults with hematologic malignancies.7,8 However, 29% of our virtual patients were unable to complete the clock-drawing test, and 46% of patients were unable to complete the caregiver-administered gait speed test. A majority of patients who were unable to complete these tests cited a lack of access to or ability to operate videoconferencing technology or lack of an available caregiver to administer the test (gait speed). More engagement with caregivers and more technical assistance could increase the ability of older patients to complete the objective performance tests developed in our study.21-23 Technologic advances in patient wearables and passive monitoring devices offer promising ways of remotely measuring objective performance tests without the need for videoconferencing with staff or for administration by caregivers. Such technology could facilitate home-based interventions that target mobility and cognition, such as virtual exercise programs for cancer survivors.6,24,25
Acknowledgments: This work was supported by the Harvard Translational Research in Aging Training Program through National Institute on Aging, National Institutes of Health (NIH), grant T32AG023480 (C.D.), the Dana-Farber/Harvard Cancer Center Specialized Program of Research Excellence in Multiple Myeloma through National Cancer Institute, NIH, grant P50 CA100707 (C.D.), the Boston Claude D. Pepper Older Americans Independence Center through National Institute on Aging, NIH, grant P30 AG031679 (C.D.), and the Older Adult Hematologic Malignancy Program through the Murphy Family Fund from the Dana-Farber Cancer Institute (G.A.A.).
Contribution: All authors designed the study; N.E.B. and T.H. collected data; T.J. analyzed data; and all authors interpreted data and prepared the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gregory A. Abel, Older Adult Hematologic Malignancy Program, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; e-mail: gregory_abel@dfci.harvard.edu.
References
Author notes
Preliminary results presented at the 78th Annual Meeting of the American Geriatrics Society, 13-15 May 2021, and at the 63rd Annual Meeting of the American Society of Hematology, Atlanta, GA, 10-14 December 2021.
Data and protocol requests will be considered on a case-by-case basis and in accordance with the regulations of the Dana-Farber Harvard Cancer Center Office for Human Research Studies. Please contact the corresponding author: gregory_abel@dfci.harvard.edu.
The full-text version of this article contains a data supplement.