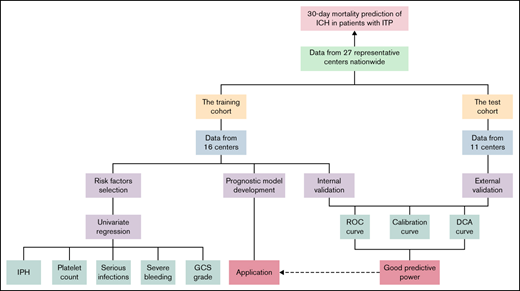
Key Points
Intraparenchymal hemorrhage, platelet count, serious infections, severe bleeding events, and Glasgow coma scale predict poor prognosis.
A prognostic model was developed and validated, and an application was established.
Abstract
Intracranial hemorrhage (ICH) is a rare and life-threatening hemorrhagic event in patients with immune thrombocytopenia (ITP). However, its mortality and related risk factors remain unclear. Herein, we conducted a nationwide multicenter real-world study of ICH in adult ITP patients. According to data from 27 centers in China from 2005 to 2020, the mortality rate from ICH was 33.80% (48/142) in ITP adults. We identified risk factors by logistic univariate and multivariate logistic regression for 30-day mortality in a training cohort of 107 patients as follows: intraparenchymal hemorrhage (IPH), platelet count ≤10 × 109/L at ICH, a combination of serious infections, grade of preceding bleeding events, and Glasgow coma scale (GCS) level on admission. Accordingly, a prognostic model of 30-day mortality was developed based on the regression equation. Then, we evaluated the performance of the prognostic model through a bootstrap procedure for internal validation. Furthermore, an external validation with data from a test cohort with 35 patients from 11 other centers was conducted. The areas under the receiver operating characteristic (ROC) curves for the internal and external validation were 0.954 (95% confidence interval [CI], 0.910-0.998) and 0.942 (95% CI, 0.871-1.014), respectively. Both calibration plots illustrated a high degree of consistency in the estimated and observed risk. In addition, the decision curve analysis showed a considerable net benefit for patients. Thus, an application (47.94.162.105:8080/ich/) was established for users to predict 30-day mortality when ICH occurred in adult patients with ITP.
Introduction
Immune thrombocytopenia (ITP) is an acquired isolated thrombocytopenia with a platelet count of <100 × 109/L, characterized by platelet destruction and impaired platelet production.1-4 ITP can occur in all age ranges, with an incidence of 3.9 to 6.1 per 1 000 000 people every year, with 2 peaks, one during childhood and one at >60 years of age.5,6 Compared with the short-term and self-limited disease courses in children, ITP is more likely to be persistent or chronic in adults due to different pathogeneses.1,7
Bleeding has been considered one of the important clinical outcomes for ITP patients.8 Although the mortality rate of ITP is only slightly higher than that of the general population, severe hemorrhagic events such as intracranial hemorrhage (ICH) are often considered to be associated with a poor prognosis.9-12 According to previous reports, ICH occurs in approximately 1% of ITP patients with a mortality of 24.0% to 31.2%.9,13-15 Lower platelet count, prior bleeding events, and antecedent head trauma have been shown to be associated with the development of ICH in pediatric and adult patients.13-17 Meanwhile, prior hemorrhagic events and newly diagnosed ITP were related to death in a cohort of 40 children.14 However, to date, risk factors for death from ICH in adults with ITP remain unclear.
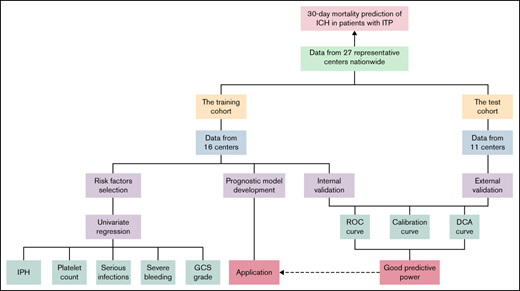
We conducted a nationwide multicenter real-world study to identify risk factors for death within 30 days following ICH in ITP patients. To the best of our knowledge, this is the largest cohort of ICH in ITP to date. Furthermore, we developed and validated a prognostic model. An application was established to predict the outcome of ICH in adult patients with ITP.
Methods
Patient selection and study design
We conducted a nationwide, multicenter, real-world study to collect data from patients with ITP who experienced ICH between 2005 and 2020. A total of 27 representative hospitals participated in this study, with a training cohort from 16 centers and a test cohort from another 11 centers. The specific list of centers is summarized in supplemental Table 1. We took full account of the geographical diversity when dividing the study population. The specific inclusion criteria were as follows: 1) age >18 years old; 2) a diagnosis of primary ITP; and 3) ICH occurring after the diagnosis of ITP. Patients newly diagnosed with ITP at the onset of ICH were excluded. All patients included in our study had a follow-up period of ≥30 days following ICH. For each patient experiencing ICH enrolled, a control case from the same center matched for time to ITP diagnosis (±1 year) and ITP duration (±180 days) was selected. Data including demographic information, comorbidities, disease characteristics for ITP, antecedent events, and clinical features of ICH were recorded in detail. To ensure accuracy, relevant information of each individual was extracted from the electronic medical record system and checked by another researcher independently.
This study was conducted in strict accordance with the Declaration of Helsinki and was approved by ethics committees or central institutional review boards of the Peking University People's Hospital and all other hospitals involved.
Definitions and evaluations
Diagnoses for primary ITP were identified based on the current international criteria.3,18-20 Secondary ITP patients were not included in our study.14,16 According to the duration of ITP, disease courses were divided into newly diagnosed (<3 months), persistent (3 to 12 months), and chronic (>12 months).3,20 The severity of hemorrhagic events before ICH was graded by the extent of involvement: mild (skin manifestations or no bleeding), moderate (visible mucosal bleeding), and severe (organ or internal mucosal bleeding).16,21 A life-threatening bleeding event could be diagnosed with hemorrhagic shock or hemoglobin loss >2 g/dL within 1 day.16
ICH refers to any hemorrhage involving the intracranial vault, including intraparenchymal (IPH), subarachnoid (SAH), subdural, and epidural locations.22,23 The diagnosis of ICH was based on imaging evidence from computed tomography or magnetic resonance imaging. Impairment of consciousness on admission was evaluated by the Glasgow Coma Scale (GCS): mild (13 to 14), moderate (9 to 12), or severe (3 to 8).24 Previous infections referred to infections occurring 1 month before ICH or at the last follow-up. Serious infections refer to those requiring IV infusion of antibacterial, antifungal, or antiviral drugs for treatment, ranging from grade ≥3 according to the Common Terminology Criteria for Adverse Events grading scale (version 5.0).25 In our study, hypertension referred to systolic blood pressure ≥160 mm Hg and/or diastolic blood pressure ≥100 mm Hg of hypertension grade ≥2.26 The diagnostic criteria for diabetes mellitus were fasting blood glucose ≥7.0 mmol/L or glycated hemoglobin ≥6.5%.27 Impaired renal function referred to a serum creatinine ≥200 μmol/L, undergoing long-term dialysis, or a history of renal transplantation.15 A platelet count <30 × 109/L after 28 days of treatment was considered glucocorticoid resistance.28
Statistical analysis
In our study, the distribution of categorical variables was recorded in counts (proportions), which were compared by Pearson's χ2 test and Fisher's exact test. Continuous variables were expressed as the median (interquartile range) and were compared by the Mann-Whitney U test. Statistically significant differences were defined as P < 0.05 (2-sided). We conducted logistic regression analysis with Firth’s penalized likelihood to select variables included in the prognostic model. Thus, the prognostic model was developed using backward stepwise logistic regression according to the Akaike information criterion. Internal validation of the model was performed by repeating the bootstrap method 1000 times for the training cohort. Furthermore, the test cohort from geographically independent centers was used to implement the external validation. The predictive value of this prognostic model was assessed by its discriminative power (via area under the curve [AUC]), calibration power (via calibration plots), and net benefit (via decision curve analysis). All of the above statistical work was calculated using SAS 9.4 for Windows and R software 4.0.3.
Results
Baseline characteristics
In total, 142 adult ITP patients with ICH were enrolled in our study. All ICHs were symptomatic; in other words, there was no subclinical ICH detected incidentally by magnetic resonance imaging. We summarized the clinical features of the patients in Table 1 and compared the differences between the training and test cohorts. Among patients with ICH, the median age was 53 years (interquartile range, 35 to 64 years), and 33.80% (48/142) of patients died within 30 days. There were no significant differences in sex, age, comorbidities, platelet count at ITP diagnosis, ITP duration, previous treatments, or events before ICH in the training and test cohorts. The 30-day mortality rates for the training and test cohorts were 36.45% (39/107) and 25.71% (9/35), respectively, with no significant difference (P = 0.244). In addition, 142 control cases of ITP adults without ICH were also enrolled in our study. The baseline clinical features are summarized in supplemental Table 2.
Clinical features of ICH
The median platelet count at the occurrence of ICH was 7 × 109/L in our study cohort. Among them, 65.7% (90/142) of patients had platelet counts of 10 × 109/L or less when ICH occurred.
In our study, 129 patients had specific bleeding site records. Among them, bleeding events in 29 patients occurred in >1 intracranial site. The parenchyma was the most commonly affected area (58.14% [75/129]). In addition, the percentages of patients suffering from SAH, subdural, and epidural were 23.26% (30/129), 21.71% (28/129), and 2.33% (3/129), respectively. A total of 44.3% of our study cohort (63/142) had an abnormal GCS score on admission. A total of 14.79% (21/142) of patients had combined serious infections. A total of 71.43% (15/21) of them were treated with glucocorticoids, and 14.28% (3/21) were on rituximab. The detailed clinical features at ICH in the training and test cohorts are listed in Table 1.
Risk factors for developing ICH
We depicted the receiver operating characteristic (ROC) curve to determine the association between platelet count at ITP diagnosis and ICH. As shown in supplemental Figure 1, the baseline platelet count was significantly associated with the occurrence of ICH in adult patients with ITP (P < 0.001; AUC = 0.702; 95% CI, 0.641-0.763). Accordingly, a platelet count ≤10 × 109/L at initial diagnosis was identified as the optimal discriminant threshold of a higher ICH risk with a Youden index of 0.338.
Furthermore, we conducted a logistic regression analysis to identify other risk factors for developing ICH. According to the univariate and multivariate analyses, platelet count ≤10 × 109/L, preceding severe bleeding events, head trauma, or surgery were independent factors for adult ITP patients developing ICH.
Variable selections for the prognostic model
In total, 107 patients were included in the training cohort. We performed a univariate logistic regression analysis to identify potential predictors to include in the prognostic model. IPH, SAH, platelet count at ICH ≤10 × 109/L, GCS score on admission, a combination of serious infections, grade of preceding bleeding events, and absence of head trauma were selected as potential predictors of 30-day mortality of ICH with a P < 0.10 (Table 2).
Development of the prognostic model
Multivariate logistic regression analysis was performed in the training cohort. Nine patients were missing data about the specific locations of ICH, and thus, 96 patients were ultimately included to develop the prognostic model. As shown in Table 2, IPH (P = 0.001), platelet count at ICH ≤10 × 109/L (P = 0.045), preceding severe bleeding events (P = 0.014), GCS between 9 and 12 (P = 0.016), and GCS ≤8 (P = 0.04) were proven to be independent risk factors for 30-day mortality of ICH. In addition, although statistically nonsignificant (P = 0.083), combined serious infections might also affect the 30-day prognosis of patients in clinical practice and were also included in the prognostic model. The coefficients of each factor in the 30-day mortality prediction model were recorded as the β-value of the regression equation.
Furthermore, to demonstrate the applicability of our prognostic model among different ages, we conducted a multivariate analysis adjusted for age. As shown in supplemental Table 4, the risk factors involved in the prognostic model were all related to the 30-day outcomes of patients of different ages.
Internal validation of the prognostic model
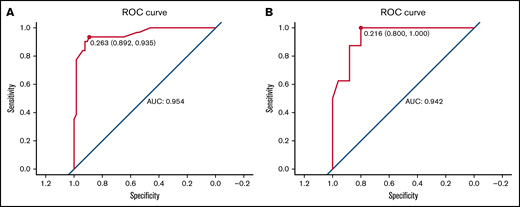
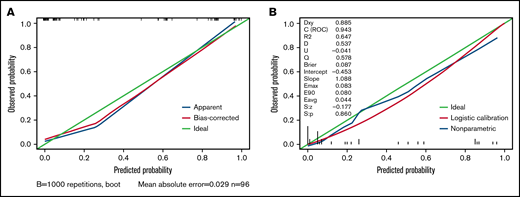
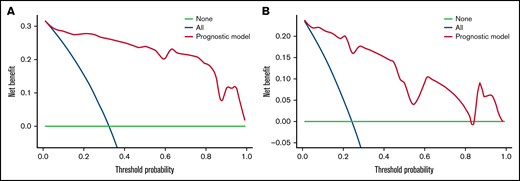
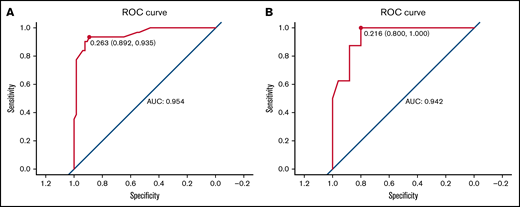
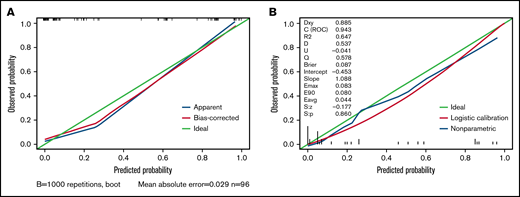
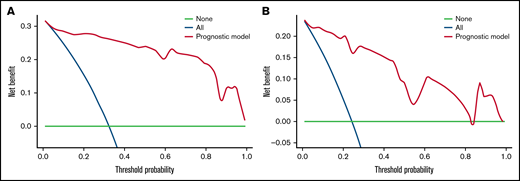
We carried out internal validation by 1000 bootstrap replicates in the training cohort. Accordingly, we plotted a ROC curve (Figure 1A). The AUC was 0.954 (95% CI, 0.910-0.998), which illustrated the good discrimination power for predicting 30-day mortality of ICH in adult patients with ITP. Meanwhile, the calibration plot demonstrated high consistency between the estimated and actual risks of 1-month death (Figure 2A), which further demonstrated the prognostic efficacy of this model. Moreover, we generated a decision curve analysis curve to determine whether the model could provide clinical benefit for ITP patients suffering from ICH. As shown in Figure 3A, the prognostic model contributed to considerable net benefits.
ROC curve of the prognostic model in the training and test cohorts. (A) ROC curve of the prognostic model for the training. The area under the curve (AUC) was 0.954 (95% CI, 0.910-0.998). (B) ROC curve of the prognostic model for the test cohort. The AUC was 0.942 (95% CI, 0.871-1.014).
ROC curve of the prognostic model in the training and test cohorts. (A) ROC curve of the prognostic model for the training. The area under the curve (AUC) was 0.954 (95% CI, 0.910-0.998). (B) ROC curve of the prognostic model for the test cohort. The AUC was 0.942 (95% CI, 0.871-1.014).
Calibration plot of the prognostic model in the training and test cohorts. (A) Calibration plot of the prognostic model for the training cohort. (B) Calibration plot of the prognostic model system for the test cohort. The x-axis plots the predicted 30-day mortality of ICH in adult patients with ITP; the y-axis plots the observed 30-day mortality in our study. A 45° diagonal line represents the ideal calibration plot.
Calibration plot of the prognostic model in the training and test cohorts. (A) Calibration plot of the prognostic model for the training cohort. (B) Calibration plot of the prognostic model system for the test cohort. The x-axis plots the predicted 30-day mortality of ICH in adult patients with ITP; the y-axis plots the observed 30-day mortality in our study. A 45° diagonal line represents the ideal calibration plot.
Decision curve analysis of the prognostic model in the training and test cohorts. (A) Decision curve analysis of the prognostic model for the training cohort. (B) Decision curve analysis of the prognostic model for the test cohort. Black line: assuming no patient died within 30 days. Gray line: assuming all patients died within 30 days. These 2 lines served as references.
Decision curve analysis of the prognostic model in the training and test cohorts. (A) Decision curve analysis of the prognostic model for the training cohort. (B) Decision curve analysis of the prognostic model for the test cohort. Black line: assuming no patient died within 30 days. Gray line: assuming all patients died within 30 days. These 2 lines served as references.
External validation of the prognostic model
To further evaluate the predictive power of our prognostic model, we performed independent external validation with a test cohort from 11 other geographically independent centers. Four patients were missing data on the ICH type, so 31 cases were used for the external validation process. As shown in Figure 1B, the AUC of the ROC curve in the test cohort was 0.942 (95% CI, 0.871-1.014), which further proved the discrimination power of the prognostic model. The calibration efficacy was further illustrated as a highly consistent calibration plot in the test cohort (Figure 2B). Moreover, patients in the test cohort also attained net clinical benefits from this prognostic model (Figure 3B).
Establishing an application based on the prognostic model
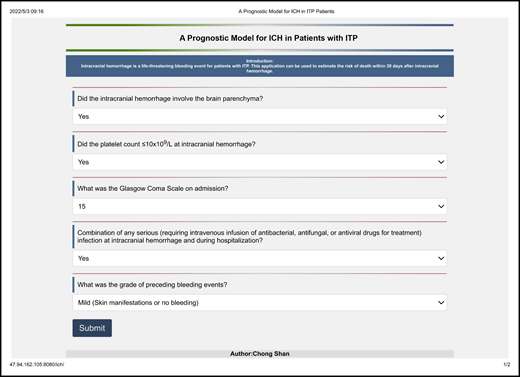
Based on the analysis above, we believe the prognostic model has the considerable predictive ability for 30-day mortality of ICH in ITP patients. Thus, we established an application (47.94.162.105:8080/ich/) based on this prognostic model (Figure 4). Users could log on to the website to predict patient outcomes when ICH occurred in adult patients with ITP.
An application (47.94.162.105:8080/ich/) to predict the 30-day mortality of intracranial hemorrhage (ICH) in ITP patients. Users can visit the site and fill in these associated clinical features. After submission, the application can automatically output the patient's risk of death within 30 days following ICH in the form of a percentage.
An application (47.94.162.105:8080/ich/) to predict the 30-day mortality of intracranial hemorrhage (ICH) in ITP patients. Users can visit the site and fill in these associated clinical features. After submission, the application can automatically output the patient's risk of death within 30 days following ICH in the form of a percentage.
Discussion
ICH has been regarded as a severe and fatal complication in patients with ITP, the 30-day mortality of which is usually a clinical outcome of concern to physicians.8,9,29 It has been demonstrated that ICH mortality was significantly higher in patients with coagulation disorders than in those without coagulation disorders.30 However, probably due to its low incidence, no large-scale clinical studies have thus far confirmed the 30-day mortality and associated risk factors in adult ITP. We conducted a nationwide multicenter real-world study with 142 samples to address this research gap. The mortality rate for patients with ICH was previously reported to be 25% to 52%, similar to the mortality rate of 33.8% for ITP patients in this study.31-33 We identified 5 risk factors (IPH, platelet count ≤10 × 109/L at ICH, a combination of serious infections, grade of preceding bleeding events, and GCS level on admission) associated with death. Accordingly, a prognostic model of 30-day mortality was developed and validated. Thus, we have developed an application in the form of a website where users can log in to predict the risk of death of ICH in ITP patients.
The platelet count in patients with ITP is always a concern. In our study, we identified an association between a low platelet count (≤10 × 109/L) and the development and prognosis of ICH in adult ITP patients. The risk threshold was also consistent with previous reports of ICH or other major bleeding events.17,34 , -38 Meanwhile, this finding has also been observed in patients with acute leukemia.39 Previous experimental studies may provide a potential explanation for this risk threshold, reporting that platelets could maintain vascular hemostasis unless the platelet count was <7 to 8 × 109/L.40 Therefore, it is important to recognize that for patients with severe ITP (platelet count ≤10 × 109/L), intensive therapy should be adopted. This study found that patients with a previous serious bleeding event had a higher likelihood of developing ICH and an even worse prognosis. Platelet microparticles, vesicles derived from platelet membranes, were reported to be 50- to 100-fold more procoagulant than the surface of activated platelets and to be significantly higher in patients with ITP.41 However, the concentrations of platelet microparticles are different in ITP patients with and without hemorrhage, which probably accounts for the risk and prognosis of bleeding.42 To date, several scoring systems have been proposed to predict the future risk of bleeding in ITP patients.21,43,44 Therefore, we believe that more aggressive interventions are necessary for patients at higher risk of bleeding.
According to large-scale epidemiological surveys, the short-term mortality of IPH is 25% to 48%.45,46 More importantly, thrombocytopenia might contribute to a progressive hemorrhage of IPH.47 In fact, thrombocytopenia has also been found to be a predictor of a poor outcome in spontaneous IPH.48
In addition, there is a great deal of evidence that the GCS score could be an independent predictor of early death in patients with ICH.49 As reported, a lower GCS score might indicate a worse ICH and a greater hemorrhage volume, which could explain the association with a poor prognosis.50,51
Infection complications were reported to occur in 20% to 31% of patients with ICH as a risk factor for a poor prognosis.52-55 In fact, acute brain injury could lead to immunosuppression because of the dysregulation of brain–immune interactions, which accounts for the high incidence and poor prognosis of coinfection in ICH. Meanwhile, it has been noted that the use of glucocorticoids and rituximab in ITP patients exposes them to a higher risk of infection.56,57 The high morbidity and mortality of pneumonia in ITP patients have also been demonstrated, as in our previous study.58 Evidence has also accumulated about the association between infections and coagulation dysfunction.59,60 Thus, we speculated that ITP and infections might interact with each other. This reminded us again of the importance of combating infection in patients with ITP.
We found that cranial trauma was a risk factor for the development of ICH, consistent with previous conclusions.13,15,16 However, we found no deaths in any patients (n = 12) with ICH secondary to prior trauma, which might suggest the severity of spontaneous ICH in patients with ITP.
Notably, older age (≥70 years) was related to 30-day mortality of ICH according to the univariate analysis; although we did not find it to be an independent risk factor, which might be explained by the association between older age and IPH, a combination of serious infections, and GCS on admission.61-63 Due to sample size limitations, we did not stratify patients to analyze the differences in risk factors for death among patients of different ages. In addition, the use of medications interfering with hemostasis was previously reported to affect the outcome of patients with ICH.64,65 However, due to the thrombocytopenia of our study population, only 12 patients reported the use of medications interfering with hemostasis, so it was difficult to identify their impact on the prognosis of ICH.
Recent consensus recommended a combination of initial treatment (eg, steroids and IV immunoglobulin) and platelet transfusion as an emergency treatment for patients with active, significant bleeding. TPO-RA and other options should be considered, especially for patients without significant response to first-line therapy.18 Thereby, an individualized assessment for disease severity and prognosis stratification may contribute to such therapeutic decision-making. For patients who have developed ICH, our model may provide useful information for therapeutic options according to the estimated risk of mortality, including special treatments, the timing of interventions, and the balance of treatment benefits and costs. Notably, 66.9% of patients developing ICH in this study suffered chronic ITP, indicating the significance of subsequent treatment options. The choice of second-line therapy, as highlighted by recent guidelines, should be individualized based on multiple factors, including the duration of ITP and the frequency of bleeding episodes requiring hospitalization or rescue medication.18 Consistent with previous studies, our work found risk factors, including a platelet count <10 × 109/L, preceding hemorrhagic events, and head trauma, for the development of ICH.13 , -17,66 More importantly, we identified that a low platelet count (<10 × 109/L) and severe bleeding indicated a poor prognosis of ICH. These findings may assist with the refined management of chronic ITP. Patients with a high risk for ICH and those with higher estimated mortality once developing ICH might be considered for more intensive therapy (eg, a combination of thrombopoietin receptor agonists or rituximab with initial treatment and sometimes splenectomy18 ) to increase the platelet count.
Some limitations existed in this real-world study. First, due to the limitations of the retrospective nature of the study, some data were missing. Meanwhile, there might be some recall bias in the reporting of hemorrhagic events before ICH. Occult bleeding, for instance, microscopic hematuria and cerebral microbleeds, have been reported to be frequent in severe ITP.66,67 Therefore, the frequency and severity of antecedent hemorrhagic events were likely to be underestimated. In addition, we defined 30-day mortality as the primary outcome indicator, but other poor prognoses following ICH, including disability and neurological dysfunction, may also merit investigation. Additionally, despite having the largest population of ICH in ITP to date, our cohort sample was of a single nationality and still not large enough, which might affect the performance of the prognostic model. We expect that our prognostic model will be used in clinical practice, providing benefits to patients and further validating its predictive power.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (No. 2021YFC2500300), National Natural Science Foundation of China (No. 81970113), Key Program of National Natural Science Foundation of China (No. 81730004), and Capital Health Research and Development of Special (No. 2022-1-4082).
Authorship
Contribution: X.-H.Z. and X.-J.H. designed the study; R.-B.H., H.Z., J.-N.Z., M.H., Y.L., H.-X.Y., T.N., J.P., M.J., Y.-Q.H., J.-D.H., Z.-P.Z., L.Q., L.-S.Z., X.W., H.-Q.W., R.F., L.-H.Y., L.-M.M., S.-Q.W., P.-Y.K., W.-S.W., H.-P.S., J.S., H.-B.Z., T.-N.Z., L.-R.W., and J.-Y.Z. collected and analyzed data; Q.-S.H., H.-X.F., Y.-J.W., Y.-Y.L., Q.-F.W., Q.J., H.J., and J.L. assisted in the research; S.C., P.Z., R.-B.H., and H.Z. wrote the manuscript; and all authors critically revised, reviewed, and approved the final version of this manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xiao-Hui Zhang, No. 11 Xizhimen South Street, Xicheng District, Beijing, China; e-mail: zhangxh@bjmu.edu.cn; Xiao-Jun Huang, No. 11 Xizhimen South Street, Xicheng District, Beijing, China; e-mail: xjhrm@medmail.com.cn.
References
Author notes
S.C., P.Z., R.-B.H., and H.Z. contributed equally to this study.
Data from this study can be obtained by sending an e-mail to the corresponding authors (zhangxh@bjmu.edu.cn or xjhrm@medmail.com.cn). Some of the data from this study have been used to identify risk factors of ICH in ITP patients. The results have been published in: Risk stratification and outcomes of intracranial hemorrhage in patients with immune thrombocytopenia under 60 years of age. Platelets 2021;32(5):633-641. doi: 10.1080/09537104.2020.1786042.
The full-text version of this article contains a data supplement.