Key Points
Clinical diagnosis of VITT often leads to disease overcall in Canada because most suspected patients are negative for anti-PF4 antibodies.
EIAs have an excellent sensitivity and specificity for detecting anti-PF4 VITT antibodies and play an important role in diagnosis of VITT.
Abstract
Vaccine-induced immune thrombotic thrombocytopenia (VITT) is a rare but serious adverse syndrome occurring 5 to 30 days after adenoviral vector COVID-19 vaccination. Therefore, a practical evaluation of clinical assessments and laboratory testing for VITT is needed to prevent significant adverse outcomes as the global use of adenoviral vector vaccines continues. We received the clinical information and blood samples of 156 patients in Canada with a suspected diagnosis of VITT between April and July 2021. The performance characteristics of various diagnostic laboratory tests were evaluated against the platelet factor 4 (PF4)-14C-serotonin release assay (SRA) including a commercial anti-PF4/heparin immunoglobulin G (IgG)/IgA/IgM enzyme immunoassay (EIA, PF4 Enhanced; Immucor), in-house IgG-specific anti-PF4 and anti-PF4/heparin-EIAs, the standard SRA, and the PF4/heparin-SRA. Of those, 43 (27.6%) had serologically confirmed VITT-positive based on a positive PF4-SRA result and 113 (72.4%) were VITT-negative. The commercial anti-PF4/heparin EIA, the in-house anti-PF4-EIA, and anti-PF4/heparin-EIA were positive for all 43 VITT-confirmed samples (100% sensitivity) with a few false-positive results (mean specificity, 95.6%). These immunoassays had specificities of 95.6% (95% confidence interval [CI], 90.0-98.6), 96.5% (95% CI, 91.2-99.0), and 97.4% (95% CI, 92.4-99.5), respectively. Functional tests, including the standard SRA and PF4/heparin-SRA, had high specificities (100%), but poor sensitivities for VITT (16.7% [95% CI, 7.0-31.4]; and 46.2% [95% CI, 26.6-66.6], respectively). These findings suggest EIA assays that can directly detect antibodies to PF4 or PF4/heparin have excellent performance characteristics and may be useful as a diagnostic test if the F4-SRA is unavailable.
Introduction
Vaccine-induced immune thrombotic thrombocytopenia (VITT) is a rare but serious adverse event associated with vaccines for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), including the ChAdOx1 nCoV-19 (Oxford-AstraZeneca; AZ) and the Ad26.COV2.S vaccine (Johnson & Johnson).1-4 VITT is characterized by moderate to severe thrombocytopenia and arterial and/or venous thrombosis 5 to 30 days after vaccination with unusual manifestations, such as cerebral venous sinus thrombosis.1 -3,5 Recent updates on the approximate risk of VITT after an AZ or Johnson & Johnson vaccine are reportedly 1/90 0006 and 1/260 000,7 respectively; or roughly 1/100 000 for both. Although the suspension of adenoviral vector vaccines for COVID-19 has led to a decline in VITT across Canada and other parts of the world, many low- and middle-income countries continue to administer these vaccines because of their stability and favorable storage conditions. Because VITT remains a global health problem, a fulsome description of the diagnostic accuracy of clinical and laboratory evaluations for VITT is needed to uphold vaccine safety worldwide.
The immunological mechanism of VITT resembles an adverse reaction to the common anticoagulant heparin, which is known as heparin-induced thrombocytopenia (HIT).8,9 HIT develops when immune complexes formed of immunoglobulin (IgG) antibodies and platelet factor 4 (PF4)/heparin interact with platelet surface FcγRIIa receptors, triggering platelet activation.10 Similarly, VITT is caused by platelet-activating anti-PF4 IgG antibodies, but without previous exposure to heparin.11,12 We recently confirmed the presence of highly reactive anti-PF4 antibodies in patients with VITT that, like HIT,13 bind to conserved sites on PF4 and cause platelet activation and thrombosis.14
VITT and HIT are diagnosed using similar laboratory tests due to their similar pathophysiologies.15 These include enzyme immunoassays (EIAs) to detect anti-PF4 antibodies followed by functional assays that confirm their platelet-activating ability. Initial experience using the standard 14C-serotonin release assay (SRA) for VITT diagnosis showed a decrease in antibody-mediated platelet activation in the presence of heparin thereby producing frequent false-negative results.5 It was then discovered that the sensitivity of platelet functional assays, including but not limited to the SRA, heparin-induced platelet activation assay (HIPA), and p-selection expression assay (PEA),16 could be increased with the addition of exogenous PF4 rather than heparin to the test system.5,15 Therefore, PF4-enhanced platelet functional assays are currently considered the most accurate diagnostic assays for VITT.5,15,16
From March 2021 to January 2022, more than 3 million Canadians received the AZ COVID-19 vaccine.17 The prompt recognition of clinical signs that might indicate VITT is important to prevent severe morbidity and mortality.8,18 Clinicians may be prone to overcalling VITT (as is the case with HIT)19 or under calling VITT because the signs of thrombocytopenia and thrombosis are fairly nonspecific. We evaluated the clinical diagnostic criteria for VITT using a practical approach based on comprehensive cohort of patients with suspected VITT in Canada. We also describe the performance characteristics of various HIT laboratory assays for the diagnosis of VITT.
Materials and methods
Study population
Blood samples used for this study were obtained from patients whose medical requisitions were received by the McMaster Platelet Immunology Laboratory from various referring centers across Canada for patients suspected of having VITT. Requisitions sent in by physicians included the following data: sample collection date, vaccine type (dose and date), description of symptoms (onset date), platelet counts, thrombosis type, and any heparin treatment (exposure date). All patients received 1 dose of the following vaccinations against COVID-19, including AZ Vaxzevria (ChAdOx1 nCoV-19), Johnson & Johnson (Ad26.COV2.S), Pfizer-BioNTech (BNT162b2), and Moderna Spikevax (mRNA-1273). VITT diagnosis was confirmed using the functional PF4-SRA, with no previous exposure to heparin.20 This study was approved by the Hamilton Integrated Research Ethics Board.
Immunoassays for the detection of anti-PF4 antibodies
Testing for IgG/A/M anti-PF4/heparin antibodies was performed using a commercially available EIA (LIFECODES PF4 Enhanced assay; Immucor GTI Diagnostics, Waukesha, WI, USA; positive optical density [OD]405nm ≥ 0.4) per the manufacturer’s instructions.21 Human recombinant PF4 used in our immunoassays, as well as our platelet functional assay, was purified in-house as previously described.13 Subsequently, an in-house IgG-specific anti-PF4-EIA and anti-PF4/heparin-EIA were performed on all samples as previously described.21 Briefly, patient sera were incubated in 96-well Maxisorp plates (Thermo Fisher Scientific, Waltham, MA, USA) coated with 30 μg/mL recombinant PF4 (or with 30 μg/mL recombinant PF4 and 0.5 U/mL heparin [Pfizer, New York, NY, USA]) in bicarbonate buffer (pH 9.6) overnight at 4°C. Plates were then washed twice with phosphate-buffered saline (PBS) with 0.05% Tween-20 then PBS alone and blocked in PBS supplemented with 3% bovine serum albumin (BSA; MilliporeSigma, St Louis, MO, USA) at room temperature for 2 hours. Plates were washed again followed by the addition of 1/50 dilutions of patient sera in PBS supplemented with 1% BSA in duplicate for 1 hour at room temperature. Bound human IgG antibodies were detected with alkaline phosphatase–conjugated goat anti-human IgG (Fc specific, Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) diluted 1/3000 in PBS supplemented with 1% BSA. After washing, 1 mg/mL p-nitrophenylphosphate (MilliporeSigma) substrate was added and ODs were measured using a BioTek 800TS microplate reader (Agilent Technologies, Santa Clara, CA, USA) at 405 nm to determine the presence of anti-PF4 or anti-PF4/heparin antibodies in patient sera (positive OD405nm ≥ 0.45).
Functional platelet activation assays
All samples were tested for platelet activation using the PF4-SRA with increasing concentrations of exogenous PF4 (0, 25, 50, 100 μg/mL; positive ≥ 20% 14C-serotonin release).20,22 Blood samples were also tested for platelet activation in the standard SRA in the presence of therapeutic (0.1-0.3 U/mL) and high (100 U/mL) doses of unfractionated heparin (Pfizer) without exogenous PF4, as previously described.23 Platelet activation was also assessed in a PF4/heparin-SRA, which tests blood samples in the presence of 0.5 U/mL unfractionated heparin and 10 μg/mL recombinant PF4.24 Each assay was performed with an anti-human CD32 Fc receptor-blocking monoclonal antibody (IV.3) to confirm FcγRIIa involvement in platelet activation.
Statistical analysis
GraphPad Prism version 9.1.2 for Mac OS software (GraphPad Software, San Diego, CA, USA) was used to create all graphs and perform statistical analysis. Performance characteristics, including sensitivity, specificity, and confidence intervals, were calculated using Microsoft Excel for Mac version 15.37. Differences between data sets were tested for statistical significance using an unpaired t test or a testing for significant difference in medians, using R package coin. P values are reported as 2-tailed where P < .05 was considered statistically significant.
Results
Patient demographics
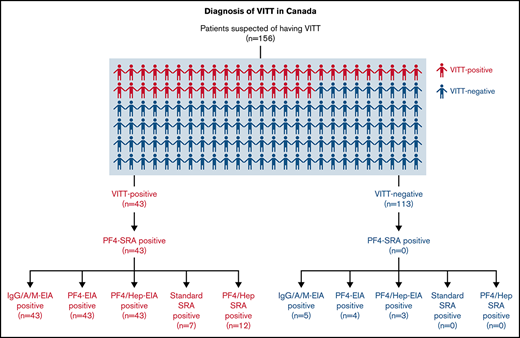
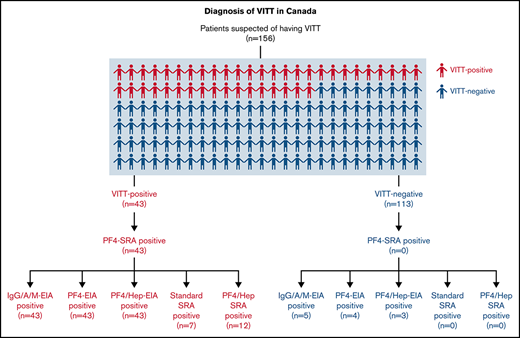
We received samples for diagnostic testing from 156 patients across Canada with clinically suspected VITT from April to July 2021. All patients included in this study had received at least 1 dose of an adenoviral vector or mRNA COVD-19 vaccine (Table 1). Presenting clinical signs and symptoms were severe headaches (22.1%), nausea (4.3%), changes in vision (4.3%), general weakness/fatigue (8.6%), shortness of breath (15.0%), limb swelling (9.3%), and pain of the chest, abdomen, or leg (28.6%). Referred patients had either thrombocytopenia (89/156, 57.1%), thrombosis (102/156, 65.4%), or both (56/156, 35.9%). A diagnosis of VITT was confirmed by a positive PF4-SRA result (≥20% 14C-serotonin release) in a patient who had received at least 1 COVID-19 vaccine dose without previous heparin exposure. Using these criteria, we classified 43 patients as VITT positive (27.6%) and 113 patients as VITT negative (72.4%).
A complete summary of clinical characteristics in both VITT-positive and VITT-negative patient cohorts can be found in Table 1. All VITT-positive patients received an adenoviral vector vaccine (42/43, 97.7%) except for 1 patient who received a Pfizer mRNA vaccine (1/43, 2.3%). The median age of the VITT-positive patient cohort was 52 years (range, 29-73 years) with a median time to symptom onset of 15 days (range, 7-61 days) post-vaccination. We found no significant difference (P = .68) between the distribution of males (51.2%) and females (48.8%) in the VITT-positive cohort. We found 30/35 (85.7%) of VITT-positive patients had evidence of thrombocytopenia and thrombosis, 2/35 (5.7%) had thrombocytopenia alone, and 3/35 (8.6%) had thrombosis alone (Table 1). The remaining 8 VITT-positive patients were not included in these calculations because of absent clinical information regarding platelet counts. Of the VITT-positive patients who had thrombosis (n = 38), 5 were missing clinical information concerning thrombosis type and thus were excluded from the following calculations. Cerebral venous sinus thrombosis (CVST; 15/38, 36.8%), deep vein thrombosis (DVT; 9/38 23.7%), and pulmonary embolism (PE; 12/38, 31.6%) were prevalent manifestations in VITT-positive patients who had thrombosis. Furthermore, we did not find a significant difference (P = .54) in the proportion of females with CVST (9/21, 42.9%) and without CVST (11/21, 52.4%) in the VITT-positive group.
The majority of referred patients were classified VITT negative (n = 113) based on serological testing using the PF4-SRA, despite presenting with thrombosis and thrombocytopenia. The median age of VITT-negative patients was 61 years (range, 19-82 years) and the median time to symptom onset was 25 days (range, 4-92 days) post-vaccination. Evidence of thrombocytopenia with thrombosis was seen in 27/91 (29.7%) VITT-negative patients. In total, thrombosis was observed in 63/99 (63.6%) VITT-negative patients who had available clinical data, including DVT (38.2%) and PE (31.7%). Additionally, CVST developed in 4.8% of VITT-negative patients, compared with 36.3% of VITT-positive patients (P < .001). The presence or absence of thrombosis was not reported for 14/113 (12.4%) VITT-negative patients.
Performance characteristics of enzyme immunoassays for VITT diagnosis
Serological testing was carried out on all referred samples using the commercial anti-PF4/heparin IgG/A/M EIA21 (Immucor) as well as in the IgG-specific in-house anti-PF4-EIA21 and in-house anti-PF4/heparin-EIA (Figure 1).21 All VITT-positive patients (43/43, 100%) were positively identified in the Immucor IgG/A/M anti-PF4/heparin EIA, IgG-specific anti-PF4-EIA, and IgG-specific anti-PF4/heparin-EIA (Table 2). When testing VITT-negative patients in the Immucor IgG/A/M anti–PF4/heparin EIA, 5/113 (4.4%) patients tested positive (Table 2) and 108/113 (95.6%) tested negative (Table 2). In the IgG-specific anti-PF4-EIA, 4/113 (3.5%) VITT-negative patients tested positive and 109/113 (96.4%) tested negative (Table 2). In the in-house IgG specific anti-PF4/heparin EIA, 3/113 (2.7%) VITT-negative patients tested positive and 110/113 (97.3%) tested negative (Table 2).
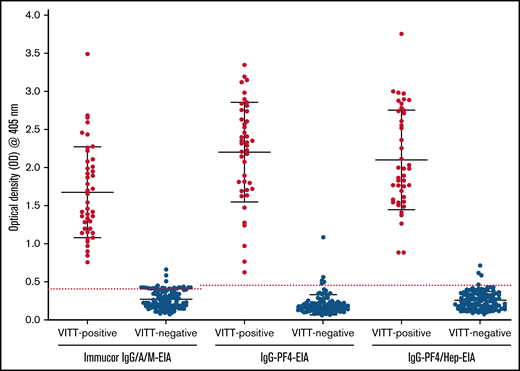
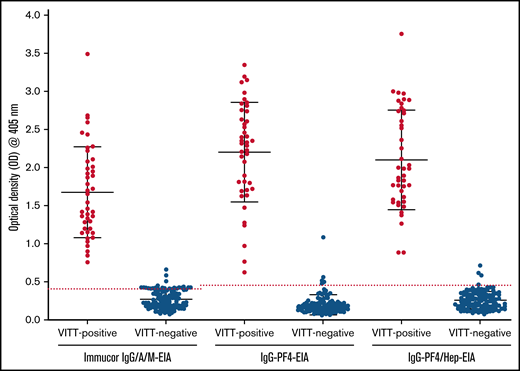
Results of 3 immunoassays for VITT antibody detection. Commercial Immucor IgG/A/M anti-PF4/heparin EIA, and the in-house IgG-specific anti-PF4-EIA and anti-PF4/heparin-EIA results for all VITT-positive patients (n = 43; PF4-SRA, ≥20% 14C-serotonin release) and VITT-negative patients (n = 113; PF4-SRA, ≤20% 14C-serotonin release). An OD405nm ≥ 0.40 is considered positive for anti-PF4 and anti-PF4/heparin antibodies in the Immucor IgG/A/M-EIA, shown as a red dotted line. An OD405nm ≥ 0.45 is considered positive for anti-PF4 and anti-PF4/heparin antibodies in the in-house IgG specific PF4/heparin-EIA and PF4-EIA, also shown as a red dotted line.
Results of 3 immunoassays for VITT antibody detection. Commercial Immucor IgG/A/M anti-PF4/heparin EIA, and the in-house IgG-specific anti-PF4-EIA and anti-PF4/heparin-EIA results for all VITT-positive patients (n = 43; PF4-SRA, ≥20% 14C-serotonin release) and VITT-negative patients (n = 113; PF4-SRA, ≤20% 14C-serotonin release). An OD405nm ≥ 0.40 is considered positive for anti-PF4 and anti-PF4/heparin antibodies in the Immucor IgG/A/M-EIA, shown as a red dotted line. An OD405nm ≥ 0.45 is considered positive for anti-PF4 and anti-PF4/heparin antibodies in the in-house IgG specific PF4/heparin-EIA and PF4-EIA, also shown as a red dotted line.
A breakdown of antibody reactivity between VITT-positive and VITT-negative patients in all 3 EIAs can be found in Figure 1. Based on our evaluation compared with PF4-SRA results, the commercial anti-PF4-enhanced IgG/A/M-EIA had a sensitivity of 100% and a specificity of 95.6%, the in-house IgG-specific anti-PF4-EIA had a sensitivity of 100% and specificity of 96.5%, and the in-house IgG-specific anti-PF4/heparin-EIA had a sensitivity of 100% and specificity of 97.4% (Table 3). The positive predictive value (PPV) and negative predictive value (NPV) were also calculated for each assay (Table 3). The commercial Immucor anti-PF4-enhanced IgG/A/M-EIA had a PPV of 93.5% and a NPV of 100%, the in-house IgG-specific anti-PF4-EIA had a PPV of 89.6% and NPV of 100%, and the in-house IgG specific anti-PF4/heparin-EIA had a PPV of 91.5% and NPV of 100%.
Performance characteristics of functional assays for platelet activation for VITT diagnosis
Patient samples were also tested in the standard SRA25 and the PF4/heparin-SRA24 to evaluate their performance characteristics in comparison with the PF4-SRA20 given the known effect of heparin on VITT antibody binding to PF4 (Table 2).5 Most VITT-positive samples had either no reactivity in the standard SRA, which measures platelet activation in the presence of increasing heparin concentrations (0, 0.1, 0.3, and 100 U/mL), or demonstrated serotonin release in buffer alone that was reduced in reactivity as heparin was added. Only 7/42 (16.7%) VITT-positive samples had similar heparin-dependent reactivity as seen with HIT patients in the standard SRA and were considered positive, whereas the remaining 35/42 (83.3%) patients were falsely negative in this assay. Based on our observations of typical heparin-dependent platelet activation, we determined that the standard SRA had a sensitivity of only 16.7% but had a specificity of 100% with a PPV of 100% and NPV of 74.3% (Table 3).
To further assess the diagnostic capacity of different platelet activating functional assays for platelet activation, a subset of VITT-positive samples (n = 26), and VITT-negative samples (n = 7) were tested in the PF4/heparin-SRA, which measures activation in the presence of therapeutic heparin (0.5 U/mL) with the addition of exogenous PF4 (10 μg/mL).23,24 The sensitivity of the PF4/heparin-SRA assay was 46.2% and the specificity was 100% based on their performance compared with the PF4-SRA (Table 3). We also determined that this assay had a PPV of 100% and a NPV of 33.3% (Table 3).
Discussion
As the reference laboratory for VITT testing in Canada, we report the efficacy of clinical diagnosis of VITT and the performance characteristics of various laboratory assays. We identified 43/156 (27.6%) total VITT-positive patients who presented with symptoms 15 days (range 7-61 days) after COVID-19 vaccination. We found no significant difference in the distribution of females in our study despite earlier reports of female over representation in VITT-positive populations,7,18,26,27 whose data may have been influenced by the demographics of early vaccine recipients. Furthermore, only 10/43 (23.3%) VITT-positive patients were female over the age of 50 years, consistent with values from other studies.7,8 Clinical manifestations in VITT-positive patients mostly included thrombocytopenia with thrombosis, but also thrombocytopenia alone and thrombosis alone. Similar findings were also noted in previous studies7,22,28 and suggest that thrombocytopenia precedes thrombosis in VITT (termed “pre-VITT”28 ), similar to the clinical profile seen in HIT patients.12 CVST, a rare manifestation of a cerebrovascular disorder, was among one of the most common thrombotic manifestations in the VITT-positive patient cohort and occurred in a similar frequency to both DVT and PE. However, we found that the mere presence of CVST is insufficient to diagnose VITT as it occurred in only one-third of VITT-positive patients and was also observed in some VITT-negative patients.
We found that 72.4% of the suspected patients in our study with a clinical presentation of thrombosis and/or thrombocytopenia did not have serologically confirmed VITT, but instead had hematological complications post-vaccination that were not caused by platelet-activating anti-PF4 antibodies. The causes of thrombosis and/or thrombocytopenia in these patients is currently unknown and the possible role of adenoviral vector vaccines in causing these complications requires further investigations. Although thrombocytopenia and/or thrombosis in VITT-positive patients occurred more significantly than in VITT-negative patients, it was still observed frequently in both cohorts, making it difficult to use these as clinical criteria to implicate or exclude VITT. In agreement with previous reports,18 we also found that although CVST was present in both the VITT-negative and VITT-positive cohorts, CVST manifested more often in patients positive for VITT. In this study, VITT-negative patients were clinically suspected of VITT because they had experienced some symptoms of thrombosis and/or thrombocytopenia following vaccination. However, many of these patients did not meet the defining criteria as outlined in the Brighton Collaboration case definition,22,29 indicative of a high clinical suspicion of VITT. Previously, our group showed that the majority of suspected HIT cases are not HIT (86.5%; false positive or true negative) and laboratory confirmation is essential to minimize overdiagnosis and overtreatment.19 Similarly, we found that the use of clinical criteria to identify VITT frequently led to disease overcall in our study. Unlike previous retrospective studies on VITT,22,30 we found that most referred patients suspected of VITT with hematological abnormalities were negative for anti-PF4 antibodies. Indeed, thrombocytopenia and/or thrombosis often presents in many clinical conditions likely coincidental with COVID-19 vaccination and serological testing for VITT was required to confirm a diagnosis in these patients.23,30
All referred patient samples were tested for VITT in 3 EIAs, which revealed positive results in the Immucor IgG/A/M anti-PF4/heparin-EIA, the IgG-specific anti-PF4 EIA, and the anti-PF4/heparin-EIA and often agreed with a positive PF4-SRA result. Previous studies have reported a reduced accuracy of commercially available HIT rapid immunoassays for identifying anti-PF4/heparin VITT antibodies, but demonstrate higher diagnostic sensitivities when evaluating EIA-based HIT assays.31,32 Similarly, we found anti-PF4 VITT antibodies were strongly reactive in the Immucor IgG/A/M anti-PF4/heparin-EIA, the in-house IgG anti-PF4-EIA, and IgG anti-PF4/heparin-EIA with high specificities for anti-PF4 VITT antibodies compared with the low specificity previously reported in HIT (∼51%).20,21,24 This is because antibodies produced by patients with HIT are polyspecific and have been shown to bind multiple sites on PF4, many of which are nonpathogenic and do not cause platelet activation or HIT.13 Studies on VITT show a restriction of antibodies to the heparin-binding site on PF4 and could account for the significant improvement in EIA specificity.14 However, standard EIAs are not able to distinguishing typical and spontaneous HIT from VITT antibodies, as recently shown by Kanak et al.33 Although platelet activation assays are the gold standard in HIT, our findings confirm previous reports31,32,34 -36 that a positive EIA result is sufficient for VITT diagnosis when aligned with clinical criteria.
Using a subset of our VITT samples, we also compared the standard SRA and the PF4/heparin-SRA to the PF4-SRA. We found these assays have a reduced diagnostic sensitivity for VITT compared with what is previously reported in HIT, possibly because of the competition between heparin and VITT antibodies for the heparin-binding site on PF4.14,23 However, some patients could have received therapy without our knowledge before specimen procurement, such as intravenous immunoglobulin, which can inhibit platelet activation in functional assays5 and may have an unknown influence on our study. Although neither the standard SRA nor the PF4/heparin-SRA appear to be suitable tests for VITT diagnosis based on our analysis, the PF4/heparin-SRA had a higher sensitivity than the standard SRA, which is consistent with a previous report on this assay involving VITT.31 We suspect the difference in reactivity in the PF4/heparin-SRA could be attributed to the addition of exogenous PF4 and heparin in this assay, similar to the PF4-SRA, whereas the standard SRA is performed only with exogenous heparin.
One limitation of this study was incomplete or absent data concerning referred patients, including D-dimer levels. This may have been due to evolving guidelines regarding VITT diagnosis at the clinical level and the variable expertise of referring physicians. This could have led to a high suspicion of VITT based on symptoms at presentation before clinical confirmation was obtained. Furthermore, VITT was relatively uncommon and unknown during early vaccine roll out, which limited physicians’ ability to identify risk factors (such as age) associated with VITT. The lack of follow-up data on VITT-negative patients represents another possible limitation of this study, which could have revealed other possible causes of thrombosis and/or thrombocytopenia. Although these data would be useful to further examine the clinical differences between VITT and other hematological disorders, it did not interfere with our evaluation of the clinical and laboratory diagnosis of VITT.
This work presents an assessment of the clinical and laboratory diagnosis of VITT using a large cohort of referred patients from across Canada, confirming previous retrospective investigations of VITT.7,8,22 However, our study is the first to our knowledge that examined VITT diagnosis from a practical perspective using real-world data. This revealed that a clinical suspicion of VITT far exceeds its expected frequency based on Brighton Collaboration criteria. Therefore, clinical evaluations alone are insufficient to distinguish between patients with VITT and those with thrombocytopenia and thrombosis syndrome unrelated to anti-PF4 antibodies in an uncontrolled hospital setting. We also found EIAs have excellent sensitivity and specificity for anti-PF4 VITT antibodies and should play an important role in diagnosis, particularly given the variable experiences of physicians in recognizing VITT in a wide range of clinical settings. Our study also suggests that traditional HIT platelet activation assays have poor sensitivity for VITT without the addition of PF4, which is demonstrated by the strong diagnostic performance of the PF4-SRA compared with the regular SRA assay. Our findings further confirm EIAs should have a central diagnostic role in laboratories with limited access to platelet functional assays. However, functional platelet activation assays with added PF4 should remain as confirmatory tests for VITT in complex cases. Additionally, the excellent negative predictive value of the EIAs suggests they could be useful to exclude VITT as a diagnosis and to track disease progression for patient management. Future studies on the diagnosis of VITT should look to substantiate the use of EIAs in combination with clinical guidelines to yield accurate testing results in a timely manner.
Acknowledgments
The authors thank Milena Hadzi-Tosev for her contribution to statistical analyses.
Funding support for this work was provided by a grant from the Canadian Institutes of Health Research (CIHR 452655) awarded to I.N. and a grant from the Public Health Agency of Canada awarded to D.M.A. and I.N.
Authorship
Contribution: A.-L.B. and M.D. developed the VITT database, analyzed and interpreted the data, and wrote the manuscript; J.G.K. and D.M.A. interpreted data and wrote the manuscript; J.W.S. and J.C.M. performed the diagnostic tests and wrote the manuscript; R.C. and N.I. analyzed the data and wrote the manuscript; I.N. designed the research, analyzed, and interpreted data and wrote the manuscript; and all authors reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ishac Nazy, HSC 3H53, 1280 Main Street West, Hamilton, ON, Canada L8S 4K1; e-mail: nazyi@mcmaster.ca.
References
Author notes
A.-L.B. and M.D. contributed equally to this study.
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author (Ishac Nazy, nazyi@mcmaster.ca) on reasonable request.