TO THE EDITOR:
Among major unmet needs in allogeneic stem cell transplantation (HSCT), the identification of a suitable donor, the control of infection complications (where cytomegalovirus [CMV] is paradigmatic), and the reduction of both disease relapse and graft-versus-host disease (GVHD) represent 4 key challenges.
Originally developed in the setting of haploidentical HSCT,1 posttransplantation cyclophosphamide (PTCy) is now an established GVHD-prophylaxis platform, preventing the risks of acute and chronic GVHD (aGVHD and cGVHD) after HLA-mismatched as well as HLA-matched unrelated and related HSCT.2-8 Despite this, the issue of high-rate infections is still a major concern.9-13
A recent CIBMTR (Center for International Blood and Marrow Transplant Research) study showed how PTCy was associated with a higher incidence of CMV infection, regardless of donor type. Moreover, CMV reactivation was associated with the abrogation of cGVHD protection in the PTCy platform.14
Herein, we aimed to investigate how the introduction of letermovir, a recently approved CMV-specific DNA-terminase inhibitor for CMV prophylaxis, could represent a turning point in real-life experience.
We analyzed all adult patients undergoing HSCT for hematologic malignancies in our center between February 2016 and February 2021. Details on conditioning regimens are shown in supplemental Table 1. GVHD prophylaxis was calcineurin-inhibitors (CNI)-free and predominantly based on PTCy with sirolimus alone for matched related donor HSCT, or in combination with mycophenolate mofetil in case of a mismatched related or unrelated donor, in accordance with local guidelines.8 Median time on GVHD prophylaxis in patients surviving more than 3 months was 189 days (interquartile range [IQR], 164-240) in the letermovir cohort and 183 days (IQR 134-230) in the no-letermovir cohort (P = .26). Population description is shown in Table 1. Letermovir-based CMV prophylaxis has been introduced in the center guidelines from 1 March 2019 on, at the daily dose of 480 mg from Day 0 to Day +100, as recommended for CMV-seropositive patients not receiving cyclosporine. Moreover, all patients received antiviral prophylaxis with acyclovir. CMV monitoring on whole peripheral blood was done every week until Day +100 and then every 2 weeks until immunosuppressive therapy withdrawal. All HSCT survivors received regular lifelong assessments. In the case of aGVHD or cGVHD, first-line systemic treatment consisted of steroids (methylprednisolone 2 mg/kg per day for aGVHD; prednisone 1 mg/kg per day for cGVHD). Agents beyond the first-line included calcineurin inhibitors, methotrexate, extracorporeal photopheresis, ruxolitinib, and ibrutinib. The local guidelines for GVHD treatment did not change from 2016 onward.
The primary endpoint of our analysis was to compare the cumulative incidence of clinically relevant CMV infection after HSCT based on PTCy GVHD prophylaxis between 105 patients receiving letermovir prophylaxis and 105 patients from a letermovir-free historical cohort. Secondary endpoints included engraftment, cumulative incidence of aGVHD and cGVHD, cumulative incidence of relapse, cumulative incidence of transplant-related mortality (TRM), progression-free survival (PFS), and overall survival (OS). Propensity score matching was adopted to reduce confounding effects in the comparison between the 2 cohorts.15 Each patient receiving letermovir was matched with a patient not receiving letermovir. Nearest-neighbor matching was used for disease, disease status, donor, and CMV serological status. To capture the overall morbidity of allogeneic HCT in the 2 cohorts, we also developed a multistate model estimating the probability of major failure events over time.16 We considered a 6-states model and 12 possible transitions. Endpoint definitions and statistical methods are detailed in the supplemental Material.
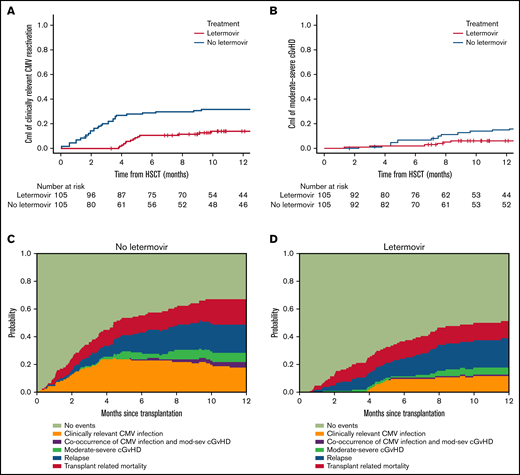
Letermovir recipients had a shorter follow-up compared with no-letermovir patients, as expected (15 months, range 3-30; and 39 months, range 3-46, respectively), due to more recent introduction of CMV-specific prophylaxis. The univariate comparison of outcomes between the 2 cohorts is shown in Figure 1 and supplemental Table 2. The 1-year cumulative incidence of clinically relevant CMV reactivation significantly differed between the 2 groups: 14% (8 to 22%) in letermovir and 32% (23 to 41%) in the no-letermovir group (P = .0005); no patients receiving letermovir developed clinically relevant CMV reactivation during drug administration. The 100-day cumulative incidence of grade 2 to 4 and 3 to 4 aGVHD were comparable in the 2 cohorts; however, we noticed a significantly reduced 1-year cumulative incidence of moderate-severe cGVHD in letermovir recipients compared with no-letermovir: 6% (2-12) and 15% (9-22), respectively, P = .01 (Figure 1; supplemental Table 2).
Comparative evaluation of clinically relevant CMV infection and moderate-severe cGVHD according to letermovir administration. The top panels show cumulative incidence (CmI) of clinically relevant CMV infection (A) and CmI of moderate-severe cGVHD (B) stratified for letermovir administration. The bottom panels show the multistate stacked transition probabilities, starting from state 1 at time of transplant, illustrating the dynamic prediction of posttransplant possible transition intensities for 2 example patients: a patient not receiving letermovir (C) and a patient receiving letermovir prophylaxis (D).
Comparative evaluation of clinically relevant CMV infection and moderate-severe cGVHD according to letermovir administration. The top panels show cumulative incidence (CmI) of clinically relevant CMV infection (A) and CmI of moderate-severe cGVHD (B) stratified for letermovir administration. The bottom panels show the multistate stacked transition probabilities, starting from state 1 at time of transplant, illustrating the dynamic prediction of posttransplant possible transition intensities for 2 example patients: a patient not receiving letermovir (C) and a patient receiving letermovir prophylaxis (D).
There was no evidence of a difference in the 1-year cumulative incidence of TRM and relapse and 1-year probabilities of OS and PFS in the 2 groups (Figure 1; supplemental Table 2). In multivariable analysis, compared with historical no-letermovir cohort (reference), patients who received letermovir experienced significantly lower hazards for clinically relevant CMV reactivation (hazard ratio [HR] 0.34; 95% confidence interval (CI), 0.20-0.70; P = .001). Moreover, letermovir administration was independently associated with moderate-severe cGVHD hazards, suggesting a protective effect (HR 0.30; 95% CI, 0.1-0.8; P = .01). Letermovir group did not show different hazards for all the other clinical outcomes analyzed. Relapse risk, as well as survival outcomes, were dependent only on disease status. Full multivariable analysis is shown in supplemental Table 3.
Figure 1 also illustrates the dynamic prediction of posttransplant possible transitions for 2 representative patients receiving or not receiving letermovir. A patient receiving letermovir has a larger probability of being free from posttransplant severe morbidities, especially concerning clinically-relevant CMV infection and moderate-severe cGVHD, with a reduction of the occurrence of both complications.
While it is well known how both GVHD and immunosuppressive treatments increase the risk of CMV reactivation, more controversial is the role of CMV in determining GVHD occurrence. Since 2010, several authors have tried to path this way, suggesting the bidirectional relationship between CMV and GVHD17 as clinically demonstrated since the beginning of the transplant activity.18 A candidate mechanism relies upon the induction of HLA class II expression on nonhematopoietic cells during viral infections resulting in an increased risk of GVHD.19 Other authors demonstrated that HLA-DP mismatch and CMV reactivation increase the risk of aGVHD independently.20 A crucial point is related to the time of CMV reactivation: the early posttransplant phase is characterized by a proinflammatory cytokine signature that contributes together with the infectious stimulus to the shaping of the immune phenotype toward a more alloreactive profile rather than a tolerogenic one. Recent insight on virome puts the accent on how some virus proteins may act through molecular mimicry or epitope spreading in the initiation or the amplification of the allogeneic reaction leading to GVHD. Herpesviridae, CMV among these, are implicated in this process.21,22 The conditional contribution of CMV reactivation early after transplantation rather than in a later phase, coupled with a more tolerogenic profile and a less inflammatory cytokines milieu, could translate into lower alloreactivity and less GVHD. Of note, the lesson learned from the pediatric field underlines how early viral infections/reactivations (CMV, Epstein-Barr virus, or adenovirus) is associated with an impaired recovery process of B cells, naïve T cells, and low T-cell receptor excision circles (a hallmark of thymic function) and higher incidence of cGVHD.23
The CIBMTR study on CMV impact on PTCy transplant unveils an interesting association between CMV reactivation and cGVHD development. It is so crucial that it nullifies the known control of cGVHD offered by PTCy.14 Irrespectively from the underlying mechanism, CMV is closely linked with GVHD, and the availability of letermovir as prophylaxis in the first 100 days after transplantation is expected to positively contribute to the overall outcomes. As recently shown by Ljungman and colleagues, letermovir exposure in the early posttransplant phase reduced mortality up to 6 months after transplantation.24 On the contrary, Terao25 and colleagues analyzed the impact of letermovir on 17 PTCy-haplo transplant recipients who received the CMV-prophylaxis in the early posttransplant phase. A higher incidence of cGVHD, but not aGVHD, was reported among PTCy-haplo recipients treated with letermovir, suggesting an early increase in the levels of HLA-DR+-activated T cells may be implicated in the development of cGVHD. This observation is interesting, although further evaluations in a larger cohort are warranted.
In our matched-pair analysis, within a CNI-free GVHD prophylaxis, we highlight the conditional benefit provided by letermovir in real-life experience, postulating for a protective effect exerted not only on clinically relevant CMV infection but also on moderate-severe cGVHD. Moreover, the dynamic multistate prediction model projects patients treated with letermovir toward a better scenario than those for patients not treated with letermovir. Although limited by the short follow-up of letermovir cohort, the retrospective and single-center nature of the comparative analysis, the limited number of events for some outcomes of interest, and the absence of a functional evaluation of the immunological determinants involved in both the CMV control and GVHD alloreactivity, these observations deserve further investigations, warranting large and multicenter comparative trials.
We speculate that if CMV reactivation may negate the cGVHD protection of PTCy, letermovir prophylaxis may restore its original benefit on cGVHD, suggesting that events occurring in the early time points after HSCT are crucial for patients’ clinical course over the long term. Further clarification of the immunological signature of these mechanisms is needed to ascertain their potential role on the path of allogeneic HSCT success.
Ethics statement: All patients were treated according to current Institutional programs upon written informed consent for transplant procedures and use of medical records for research within the noninterventional “ALMON study” approved by San Raffaele Institutional Ethical Committee on 19 October 2007.
Contribution: F.L., E.X., R.G., M.T.L.-S., F.C., and J.P. collected clinical data, interpreted results, and wrote the manuscript; F.L. performed the statistical analysis; and F.L., E.X., S.M., F.G., D.C., F.F., S.P., A.B., L.L., A.R., E.G., A.A.A., S.M., M.M., M.G.C., M.B., C.C., F.C., J.P., R.G., and M.T.L.-S. contributed to patient clinical care and data collection, critically reviewed the manuscript, and approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Maria Teresa Lupo-Stanghellini, Hematology and Bone Marrow Transplantation Unit, San Raffaele Scientific Institute, via Olgettina 60, 20132 Milano, Italy; e-mail: lupostanghellini.mariateresa@hsr.it; and Jacopo Peccatori, Hematology and Bone Marrow Transplantation Unit, San Raffaele Scientific Institute, via Olgettina 60, 20132 Milano, Italy; e-mail: peccatori.jacopo@hsr.it.
References
Author notes
F.L. and E.X. contributed equally to this study.
R.G. and M.T.L.-S. contributed equally to this study.
The datasets generated for this study are available on request to the corresponding author.
The full-text version of this article contains a data supplement.