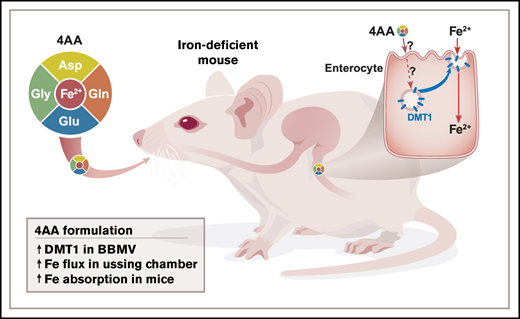
Key Points
Oral iron supplementation is usually recommended to treat iron-deficiency anemia; however, excess enteral iron has negative side effects.
We identified 4 AAs that stimulate intestinal iron absorption and may potentiate iron repletion at lower effective supplemental doses.
Abstract
Iron-deficiency anemia is common worldwide and typically treated by oral iron supplementation. Excess enteral iron, however, may cause pathological outcomes. Developing new repletion approaches is thus warranted. Previous experimentation revealed that select amino acids (AAs) induce trafficking of transporters onto the enterocyte brush-border membrane (BBM) and enhance electrolyte absorption/secretion. Here, we hypothesized that certain AAs would increase the abundance of the main intestinal iron importer, divalent metal-ion transporter 1 (DMT1), on the BBM of duodenal enterocytes, thus stimulating iron absorption. Accordingly, all 20 AAs were screened using an ex vivo duodenal loop/DMT1 western blotting approach. Four AAs (Asp, Gln, Glu, and Gly) were selected for further experimentation and combined into a new formulation. The 4 AAs stimulated 59Fe transport in mouse duodenal epithelial sheets in Ussing chambers (∼4-fold; P < .05). In iron-deprived mice, oral intragastric administration of the 4 AA formulation increased DMT1 protein abundance on the enterocyte BBM by ∼1.5-fold (P < .05). The 4 AAs also enhanced in vivo 59Fe absorption by ∼2-fold (P < .05), even when ∼26 µg of cold iron was included in the transport solution (equal to a human dose of ∼73 mg). Further experimentation using DMT1int/int mice showed that intestinal DMT1 was required for induction of iron transport by the 4 AAs. Select AAs thus enhance iron absorption by inducing DMT1 trafficking onto the apical membrane of duodenal enterocytes. We speculate that further refinement of this new 4 AA formulation will ultimately allow iron repletion at lower effective doses (thus mitigating negative side effects of excess enteral iron).
Introduction
Iron is an essential dietary constituent with well-known physiological roles in humans and other mammals1 ; logically then, iron inadequacy leads to pathophysiological outcomes. Iron deficiency is common worldwide, frequently progressing to iron-deficiency anemia (IDA). IDA is most frequently caused by dietary iron insufficiency, chronic inflammation, and the inability to assimilate adequate dietary iron due to increased demand (eg, during growth and pregnancy) or impaired absorption (eg, in Crohn’s disease).2 IDA is typically treated by oral iron supplementation, which may need to be administered several times above normal dietary intakes.3 High-dose iron supplementation, however, may cause general gastrointestinal (GI) distress/dysfunction and alteration of the gut microbiota toward a more pathogenic state.4-6 Development of improved supplementation regimens using lower effective iron doses is thus warranted.
Recently, it was noted that certain amino acids (AAs) can increase transporter abundance on the brush-border membrane (BBM) of enterocytes and thus enhance nutrient absorption.7,8 Here, we hypothesized that select AAs would increase BBM abundance of the main intestinal iron importer, divalent metal-ion transporter 1 (DMT1). AAs have been shown to enhance iron absorption, but by a different, proposed mechanism. A 1963 study demonstrated that some AAs increased 59Fe absorption from ligated duodenal loops in immobilized, anesthetized rats.9 The authors proposed that the formation of iron-AA chelates enhanced absorption. A human study provides additional evidence that AAs can influence iron absorption.10 In 150 pregnant women with IDA, an iron-AA chelate was more effective (than ferrous fumarate) at increasing blood hemoglobin (Hb) levels over a 12-week supplementation period.10
Collectively, these observations then provide the impetus for the current investigation in which we tested the hypothesis that select AAs will cause trafficking of DMT1 onto the BBM of duodenal enterocytes, thus stimulating intestinal iron transport. Accordingly, all 20 AAs were tested in blind duodenal loop assays for their ability to increase DMT1 protein abundance in BBM vesicles (BBMVs), and then confirmatory ex vivo studies were performed in the Ussing chamber followed by in vivo iron absorption studies in mice.
Methods
Ex vivo duodenal loop studies
Animal studies were approved by the University of Florida Institutional Animal Care and Use Committee. Blind loop studies were conducted on male Swiss-Webster (SW) mice (8-10 weeks old) fed a chow diet. Mice were killed, and duodenal segments (∼12 cm) were excised and flushed with Ringer’s buffer. One end was tied off, and 300 µL of an electrolyte-containing solution with or without individual AAs was added into the lumen. The buffer composition and the AA concentrations were established previously (Table 1).11,12 The opposite end of the loop was then tied off, and loops were subsequently incubated in Ringer’s buffer (pH 7.4, 296 mOsm, 37°C) bubbled with 95% O2 and 5% CO2 for 45 minutes. The luminal liquid was subsequently released, and the mucosa was then lightly scraped into 9.9 mL of lysis buffer (300 mM mannitol, 5 mM EGTA, 12 mM Tris-HCl, pH 7.1) plus 100 μL Halt Protease Inhibitor Cocktail (cat. # 78439; Thermo-Fisher Scientific).
Isolation of BBMVs and western blot analysis
Mucosal scrapes (in lysis buffer) were homogenized on ice using an IKA T25 Ultra Turrax device for 2 minutes (with 10-second breaks every 30 seconds). Five hundred µL of 1 M MgCl2 was then added, and the sample was slowly rotated at 4°C for 10 minutes. Samples were then centrifuged at 10 000 rpm for 25 minutes using a JA-12 rotor in a Beckman-Coulter ultracentrifuge. The supernatants were transferred to new tubes and centrifuged at 17 000 rpm for 40 minutes using a JA-17 rotor. The resulting pellet (containing BBMVs) was dissolved in 50 µL Ringer’s buffer plus protease inhibitors. Protein concentrations were determined using the Pierce BCA protein assay kit (cat. # 23225; Thermo-Fisher Scientific). Thirty micrograms of protein were loaded into 8% polyacrylamide gels, and separated proteins were then transferred to polyvinylidene difluoride membranes. Membranes were blocked in Odyssey blocking buffer (cat. # 927-50000; Licor) for 1 hour at room temperature and then incubated with DMT1 primary antibody (courtesy of François Canonne-Hergaux, French Institute of Health and Medical Research; Bordeaux, France) for 16 hours at 4°C (1:2000). Blots were rinsed and then incubated with donkey anti-rabbit secondary antibody (1:10 000) (cat. #925-32213; Licor) in Odyssey blocking buffer for 1 hour at room temperature. Blots were then stripped and reacted with a β-actin antibody (cat. #66009-1; Proteintech) (1:50 000) and then donkey anti-mouse secondary antibody (cat. # 926-68072; Licor) (1:10 000). Blots were imaged, and signals were quantified with a Licor Odyssey CLx immunofluorescent instrument. DMT1 protein band intensities were normalized to β-actin band intensities.
Ussing chamber flux studies
The Ussing chamber was balanced for 30 minutes prior to experimentation.11-13 Duodenal epithelial tissues from chow-fed, 8- to 10-week-old male SW mice were then mounted, equilibrated for ∼10 minutes, and paired for flux analysis based on similar conductance values. Tissues were bathed bilaterally in 10 mL of an electrolyte buffer with and without AAs (Table 2), containing 1.5 mM FeSO4, and bubbled with a 95% O2, 5% CO2 gas mixture at 37°C. The electrolyte content and AA concentrations (if used) and pH and buffering capacity of the transport solution were optimized previously.11,12 Experiments were performed with voltage clamped to 0 to minimize passive ion diffusion via paracellular flux. Blanks (500 µL) were first collected from the “cold” (nonradioactive) side. Fifteen µCi of 59Fe (as ferric chloride) (cat. # NEZ037500UC; PerkinElmer), containing an equal volume of 0.5 M N-methyl-d-glucamine, was added to either the mucosal or serosal side. At 15-minute intervals, 500 µL samples were collected from the opposite (“cold”) side, and 500 µL buffer was added back to maintain a fixed volume. At the end of each experiment, 100 µL of buffer was collected from the radioactive side of the chamber. Radioactivity was measured using a PerkinElmer Wizard2 γ Counter, and net 59Fe flux was calculated as Jnet = JmsJsm, where Jms is mucosal to serosal flux (absorption) and Jsm is serosal to mucosal flux (secretion).
Time course of iron depletion
SW mice (8 weeks) of both sexes were fed a control diet with ∼50 ppm Fe (Envigo; TD.130018) for 5 days prior to initiating experimentation. Then, the experimental group was given a low-iron diet (3 to 5 ppm Fe; Engivo; TD.120105) and housed in overhanging, wire mesh-bottomed, stainless-steel cages. The control group was given the 50 ppm Fe diet throughout and housed in conventional static cages. The time points for sacrifice were: 0.5, 1, 2, 4, 8, 10 (males only), and 14 days. BBMVs were isolated and western blots were performed to quantify DMT1 protein expression. Blood Hb content was quantified,16 and liver, kidney, and spleen nonheme iron levels were also measured using a standard colorimetric assay.17
Duodenal DMT1 and ferroportin1 (FPN1) protein expression in AA-treated, iron-deficient mice
Adult (8-9 weeks) male SW mice were fed the control diet (50 ppm Fe) for 5 days and then switched to the low-iron diet (3 to 5 ppm Fe) for another 10 days. Thereafter, mice were fasted for 2 hours and then provided the electrolyte solution with and without AAs by gavage feeding. Mice were sacrificed 1 hour later, and duodenal mucosal scrapes were collected. In the first experiment, BBMVs were purified, and in the second experiment, total membranes were purified (as described below), from 5 control and 5 4 AA-treated samples. In a third experiment, mice were gavaged with the electrolyte solution only (n = 5) or the electrolyte solution plus a mixture of 5 AAs (n = 5). The 5 AAs were selected since they did not increase DMT1 protein abundance in BBMVs in loop assays (data not shown) or in Ussing chamber experiments (Figure 1). The 5 AAs were used at the following concentrations12 (in mM): Lys (3.2), Ser (10), Thr (8), Tyr (1.2), and Val (10). BBMV proteins were probed for DMT1 expression in studies 1 and 3, while FPN1 expression was assessed in study 2. To demonstrate the validity of the FPN1 antibody (cat. #MTP11-A; α Diagnostics), intestinal samples were included from a wild-type mouse, an FPN1 knockout mouse (kindly provided by Elizabeta Nemeth, UCLA), and a mouse placental sample. Membrane proteins from duodenal scrapes and placenta were isolated by Mem-PERTM Plus membrane protein extraction kit (Thermo-Fisher Scientific). Gels were run with 20 µg of protein per lane and processed for DMT1 westerns or incubated with anti-FPN1 antibody (1:2000).
The 4 AA formulation specifically increased iron flux in duodenal epithelial organ cultures in the Ussing chamber. Net 59Fe flux and conductance were quantified in the presence and absence of combinations of AAs. Flux was significantly higher in the 4 AA group as compared with controls (A); *P < .05; n = 12 for control, n = 11 for 4 AA. Conductance was significantly lower initially and after 30 and 60 minutes in the 4 AA group compared with control fluxes (B); *P < .05, **P < .01. Flux was also measured in the presence of single AAs from the 4 AA formulation (n = 3-5) (C). 59Fe flux was then compared between 4 AAs and 3 AAs (excludes Gln) (D); n = 11 for 4 AAs, n = 7 for 3 AAs. Conductance was also measured during 59Fe flux studies with 3 AAs and 4 AAs (E); **P < .01; n = 16 for 3 AAs, n = 18 for 4 AAs. 59Fe flux was also assessed in the presence of 5 AAs that did not increase DMT1 expression in the loop assays. No change was noted when comparing to control samples (F); n = 12 for control and n = 8 for 5 AAs. These 5 AAs also tightened the mucosal barrier (G); *P < .05, **P < .01; n = 16 for control and 5 AAs. 59Fe flux data were analyzed by nonparametric Student’s t test, while conductance data were analyzed by 1-way ANOVA followed by Sidak’s multiple comparison test.
The 4 AA formulation specifically increased iron flux in duodenal epithelial organ cultures in the Ussing chamber. Net 59Fe flux and conductance were quantified in the presence and absence of combinations of AAs. Flux was significantly higher in the 4 AA group as compared with controls (A); *P < .05; n = 12 for control, n = 11 for 4 AA. Conductance was significantly lower initially and after 30 and 60 minutes in the 4 AA group compared with control fluxes (B); *P < .05, **P < .01. Flux was also measured in the presence of single AAs from the 4 AA formulation (n = 3-5) (C). 59Fe flux was then compared between 4 AAs and 3 AAs (excludes Gln) (D); n = 11 for 4 AAs, n = 7 for 3 AAs. Conductance was also measured during 59Fe flux studies with 3 AAs and 4 AAs (E); **P < .01; n = 16 for 3 AAs, n = 18 for 4 AAs. 59Fe flux was also assessed in the presence of 5 AAs that did not increase DMT1 expression in the loop assays. No change was noted when comparing to control samples (F); n = 12 for control and n = 8 for 5 AAs. These 5 AAs also tightened the mucosal barrier (G); *P < .05, **P < .01; n = 16 for control and 5 AAs. 59Fe flux data were analyzed by nonparametric Student’s t test, while conductance data were analyzed by 1-way ANOVA followed by Sidak’s multiple comparison test.
Iron absorption studies
SW mice (8-10 weeks), maintained on a chow diet (with 200 ppm Fe), were fasted for 2 hours, and then given 2.5 µCi 59Fe in control or 4 AA formulation (minus FeSO4) (Table 2), by oral intragastric gavage (300 µL total volume). Subsequently, blood was taken from the tail vein, and mice were killed 2 hours after the initial gavage. The percent of dose absorbed was calculated as ([59Fe activity in carcass + blood gastrointestinal tract/59Fe activity of initial gavage dose] × 100), and 59Fe activity was quantified in the blood (cpm/µL), and duodenum and liver (cpm/mg). An additional absorption study was also performed in male SW mice that were fed a low-iron diet (and housed in overhanging wire cages) for 10 days prior to experimentation. For this experiment, given that iron deprivation enhances iron transport, mice were killed 1 hour after gavage (with blood collected at 30 minutes). This latter study was also repeated with the inclusion of FeSO4 at 1.575, 3.15, and 6.3 mM in the gavage solution. FeSO4 was chosen as it is frequently used for human iron supplementation.18 Appropriate calculations were performed to account for the isotope dilution factor, which considered decayed radioactive iron in addition to cold iron.
Statistical analyses
Student’s t test was used when comparing 2 groups (GraphPad Prism 7). Ussing chamber flux studies were analyzed using a Student’s nonparametric t test, and conductance was analyzed by 1-way ANOVA and Sidak’s post hoc test for multiple comparisons. A nonparametric test was performed if data were not normally distributed or if there was unequal variance among groups. Western blot quantification data for the time course of iron depletion study were analyzed by Kruskal-Wallis 1-way ANOVA.
Results
Loop studies
To examine the influence of AAs on intracellular DMT1 trafficking, ex vivo duodenal loop studies followed by isolation of BBMVs and western blotting to quantify DMT1 protein expression were performed individually with all 20 AA (data not shown). The AAs were dissolved in an electrolyte-containing transport buffer. These experiments revealed large interanimal variation in DMT1 expression, irrespective of the treatment group, and as such, statistical significance was not achieved (despite n = 9 for some AAs). Nonetheless, we selected 4 amino acids (Asp, Gln, Glu, and Gly) that tended to increase DMT1 expression in BBMVs (relative to controls). Based upon our previous work with AAs and electrolyte transporters,7,8,12 we hypothesized that a mixture of these AAs could have synergistic effects. Therefore, the 4 AAs were combined into a single formulation, and additional ex vivo and in vivo analyses were performed. These experiments produced positive outcomes (as described below), thus validating the selection of these 4 AAs.
Ussing chamber flux studies
The 4 AA formulation was next tested in Ussing chambers to assess the influence on iron transport in duodenal epithelial tissue. The electrolyte-containing transport buffer composition and the AA concentrations were based upon our previously published work. 59Fe flux studies demonstrated that the 4 AAs increased iron transport (Jnet) by ∼4-fold as compared with buffer only (P < .05) (Figure 1A). Interestingly, conductance was lower in the 4 AA-exposed tissues (P < .05 at t = 0 minutes; P < .01 at 30 and 60 minutes) (Figure 1B), exemplifying a protective effect of the 4 AA formulation on barrier integrity (which could positively influence iron absorption). To further refine the 4 AA formulation, flux studies with individual AAs were performed to determine their effect on net 59Fe flux. Glutamine resulted in the lowest flux increase, so a 3 AA formulation was created that excluded glutamine (Figure 1C). Flux studies were repeated, but net 59Fe flux increase with a 3 AA or 4 AA formulation in the transport buffer did not differ (Figure 1D), but the 3 AA formulation tended to increase conductance (significant at 60 minutes) (Figure 1E). Therefore, subsequent experiments used the 4 AA formulation. In sum, the 4 AA formulation enhanced vectorial iron flux and tightened the mucosal barrier in duodenal epithelial sheets in the Ussing chamber. The next logical step was thus in vivo testing to assess physiological significance.
Iron absorption studies in iron-replete mice
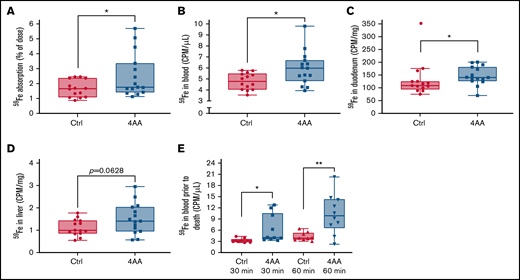
Intestinal iron absorption was measured using a well-established radiotracer assay in which 59Fe is delivered by oral, intragastric gavage, and then 59Fe activity is assessed in the blood, tissues, and carcass after the animals are killed. The percent of 59Fe dose absorbed was calculated 2 hours after delivery of the transport solution. Since 60% to 80% of the total amount of iron ultimately transferred to the body is absorbed within seconds to minutes, this experimental approach quantifies the initial (rapid) phase of iron absorption (note that a slower phase, of lesser magnitude, occurs from 12 to 48 hours after ingestion).19 The 2-hour radiotracer study in male mice demonstrated that the 4 AA formulation increased 59Fe absorption, along with 59Fe in the blood and duodenum (P < .05) (Figure 2A-C). In the liver, 59Fe activity was also seemingly higher in the 4 AA group (P = .0628) (Figure 2D). 59Fe activity in the blood was also significantly higher at 30 minutes (P < .05) and 60 minutes (P < .01) in the 4 AA group (Figure 2E). Consistent with the Ussing chamber flux data then, the 4 AA formulation stimulated iron absorption in vivo. The magnitude of the increase, however, was of questionable physiological significance. Therefore, since we surmised that the 4 AA formulation could be more effective during iron depletion, and to better model human iron supplementation, these experiments were repeated in iron-deficient mice.
The 4 AA formulation increased iron absorption in chow-fed male mice. 2.5 µCi of 59Fe in the control (Ctrl) or 4 AA formulation was administered to each mouse by oral, intragastric gavage after a 2-hour fast. Two hours later, mice were sacrificed, and 59Fe absorption was quantified (as the percentage of the dose absorbed) (A), and 59Fe activity in the blood (B), duodenum (C), and liver (D) were measured. 59Fe activity was also assessed in the blood 30 and 60 minutes after gavage (E). Data were analyzed by Student’s t test. n = 14 for control and n = 15 for 4 AAs; *P < .05, **P < .01. One statistical outlier was removed from the Ctrl data set in (C) (marked in red).
The 4 AA formulation increased iron absorption in chow-fed male mice. 2.5 µCi of 59Fe in the control (Ctrl) or 4 AA formulation was administered to each mouse by oral, intragastric gavage after a 2-hour fast. Two hours later, mice were sacrificed, and 59Fe absorption was quantified (as the percentage of the dose absorbed) (A), and 59Fe activity in the blood (B), duodenum (C), and liver (D) were measured. 59Fe activity was also assessed in the blood 30 and 60 minutes after gavage (E). Data were analyzed by Student’s t test. n = 14 for control and n = 15 for 4 AAs; *P < .05, **P < .01. One statistical outlier was removed from the Ctrl data set in (C) (marked in red).
Time-course of iron depletion
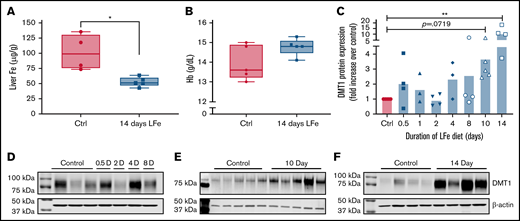
Intestinal DMT1 expression is strongly induced by iron depletion in laboratory rodents.20,21 We postulated that the 4 AAs would be most effective at enhancing iron absorption prior to maximal induction of DMT1 expression (ie, during the earlier stages of iron deficiency and prior to the onset of anemia). Therefore, to guide further intervention studies, iron status and intestinal DMT1 protein levels were quantified after varying times of dietary iron deprivation. In adult male mice, liver nonheme iron levels decreased ∼50% in the low-iron diet group (compared with controls), but only at the 14-day time point (Figure 3A). Blood Hb levels (Figure 3B; supplemental Figure 1) and spleen and kidney nonheme iron levels (not shown) did not decrease at any time points. Thus, after 14 days of dietary iron deprivation, male mice were iron-deficient but not anemic. BBMV DMT1 protein expression was highly variable in individual mice, but significant increases were noted only after 14 days of dietary iron deprivation (P < .01) (Figure 3C-F; supplemental Figure 2).
DMT1 protein expression is strongly induced in male mice by dietary iron deprivation for 14 days. Eight-week-old mice were fed a control diet (50 ppm iron) or a low-iron diet (2-6 ppm iron) for 0.5, 1, 2, 4, 8, 10, or 14 days (n = 5 for each diet at each time point). Upon sacrifice, BBMVs were isolated from duodenal scrapes, liver was collected for nonheme iron analysis, and blood was collected for Hb measurement. Liver nonheme iron content was lower after 14 days of dietary iron deprivation (A) (*P < .05; Student’s t test), but blood Hb levels were unaffected (B) (Student’s t test). Western blot analysis of proteins from duodenal BBMV preps was also undertaken. Quantification of data from multiple experiments showed that DMT1 was most strongly and reproducibly induced by 14 days of low-iron feeding (C) (n = 3-6 per group). Data were analyzed by Kruskal-Wallis 1-way ANOVA followed by Dunn’s multiple comparison test (**P < .01). Also shown are representative time course DMT1 western blots of duodenal proteins isolated from control and iron-deprived mice (D-F). Each lane of the western blot represents 1 mouse. β-actin was used as a loading control (shown below each DMT1 blot).
DMT1 protein expression is strongly induced in male mice by dietary iron deprivation for 14 days. Eight-week-old mice were fed a control diet (50 ppm iron) or a low-iron diet (2-6 ppm iron) for 0.5, 1, 2, 4, 8, 10, or 14 days (n = 5 for each diet at each time point). Upon sacrifice, BBMVs were isolated from duodenal scrapes, liver was collected for nonheme iron analysis, and blood was collected for Hb measurement. Liver nonheme iron content was lower after 14 days of dietary iron deprivation (A) (*P < .05; Student’s t test), but blood Hb levels were unaffected (B) (Student’s t test). Western blot analysis of proteins from duodenal BBMV preps was also undertaken. Quantification of data from multiple experiments showed that DMT1 was most strongly and reproducibly induced by 14 days of low-iron feeding (C) (n = 3-6 per group). Data were analyzed by Kruskal-Wallis 1-way ANOVA followed by Dunn’s multiple comparison test (**P < .01). Also shown are representative time course DMT1 western blots of duodenal proteins isolated from control and iron-deprived mice (D-F). Each lane of the western blot represents 1 mouse. β-actin was used as a loading control (shown below each DMT1 blot).
We also studied female mice to guide the selection of one sex for further testing. Blood Hb levels did not vary among any of the experimental groups (supplemental Figure 1). As in males, DMT1 expression was increased in BBMVs only after 14 days of dietary iron deprivation (P < .01) (supplemental Figure 3). Liver, kidney, and spleen nonheme iron levels were also lower at the 14-day time point (supplemental Figure 4). So, after 14 days on the low-iron diet, female mice were iron-deficient but not anemic (like males). The iron deficiency was, however, more significant in females, as exemplified by kidney and spleen iron depletion (which did not occur in males). Therefore, since we hypothesized that the influence of the 4 AAs on iron transport would be most significant during earlier stages of iron depletion, we selected males for further experimentation.
Another important consideration was when during the 2-week time course of iron depletion to test an intervention with the 4 AA formulation. At the 14-day time point, DMT1 expression was strongly upregulated in BBMV, and liver iron was reduced. At the 8-day time point, however, DMT1 was not significantly induced, nor was liver iron depleted. Iron deficiency thus develops after 8 days of dietary iron deprivation. We thus hypothesized that a 10-day time point would be an ideal time for the 4 AA intervention. Before proceeding with functional transport studies, however, it was important to confirm that the 4 AA formulation increased BBMV DMT1 protein abundance in male mice fed the low-iron diet for 10 days.
DMT1 and FPN1 protein expression in iron-deprived male mice
After 10 days of dietary iron deprivation, BBMV DMT1 protein levels trended higher (Figure 3C,E). Since intestinal iron absorption also requires an iron exporter, ferroportin 1 (FPN1), it was important to assess how the 4 AAs influenced the expression of both transporters. FPN1 protein expression was thus also assessed in total membranes isolated from duodenal scrapes. Also, to determine whether the effect of the 4 AAs on DMT1 protein abundance was specific, we gavaged mice with a mix of 5 AAs that did not increase BBMV DMT1 protein levels in our initial screening. Outcomes showed that DMT1 protein abundance increased ∼1.5-fold upon exposure to the 4 AA formulation (Figure 4A-B), while FPN1 protein expression was unaffected (Figure 4C-D). Also, the 5 AA mixture did not increase DMT1 protein levels in BBMVs (Figure 4E-F). These data thus indicated that the 4 AA formulation specifically increased the abundance of DMT1 on the BBM of duodenal enterocytes. Given the predominant role that intestinal DMT1 plays in iron absorption, the next logical step was to determine whether increased DMT1 protein levels enhanced iron absorption in iron-deficient mice.
The 4 AA formulation specifically targets DMT1. Eight-week-old male mice (n = 10) were fed a low-iron diet for 10 days. Subsequently, mice were fasted for 2 hours and then administered an electrolyte buffer with or without the 4 AAs by oral, intragastric gavage. One hour later, mice were killed, and BBMVs were isolated from duodenal scrapes for DMT1 western blots (A). DMT1 protein levels were higher in the 4 AA group (*P < .05; unpaired t test) (B). Another group of 10 mice was treated identically, and duodenal scrapes were used to isolate total membrane proteins for FPN1 western blots (C). FPN1 expression was unaffected by the 4 AAs (unpaired t test) (D). To demonstrate specificity, another group of iron-deprived mice (n = 5) was also treated with a formulation made up of 5 AAs that did not increase DMT1 protein expression in loop studies (or buffer only; n = 5). Outcomes showed that the 5 AAs did not increase DMT1 protein levels in BBMVs (unpaired t test) (E-F).
The 4 AA formulation specifically targets DMT1. Eight-week-old male mice (n = 10) were fed a low-iron diet for 10 days. Subsequently, mice were fasted for 2 hours and then administered an electrolyte buffer with or without the 4 AAs by oral, intragastric gavage. One hour later, mice were killed, and BBMVs were isolated from duodenal scrapes for DMT1 western blots (A). DMT1 protein levels were higher in the 4 AA group (*P < .05; unpaired t test) (B). Another group of 10 mice was treated identically, and duodenal scrapes were used to isolate total membrane proteins for FPN1 western blots (C). FPN1 expression was unaffected by the 4 AAs (unpaired t test) (D). To demonstrate specificity, another group of iron-deprived mice (n = 5) was also treated with a formulation made up of 5 AAs that did not increase DMT1 protein expression in loop studies (or buffer only; n = 5). Outcomes showed that the 5 AAs did not increase DMT1 protein levels in BBMVs (unpaired t test) (E-F).
Iron absorption studies in iron-deprived mice
Male mice were deprived of dietary iron for 10 days, and then an iron absorption study was performed with and without the 4 AAs added to the transport solution. Since iron deficiency increases the rate of intestinal transport (and the bulk of iron absorption thus occurs quite rapidly), we chose a 1-hour time point for this absorption study. Results showed that the 4 AA formulation increased 59Fe absorption (P < .05), and 59Fe activity in the blood was elevated at 30 minutes (P < .05) (Figure 5A,E). 59Fe activity in blood at sacrifice (P = .07) (Figure 5B), the duodenum (P = .07) (Figure 5C), and liver (P = .08) (Figure 5D) seemingly increased in the 4 AA group. The 4 AAs thus stimulated intestinal iron absorption during iron deficiency. The magnitude of increase was greater than in iron-replete mice (Figure 2). However, since the transport solution used for these studies contained only tracer amounts of iron (as 59Fe), it was important to next determine whether the 4 AAs could also enhance iron absorption when supplemental (cold) iron was simultaneously administered.
The 4 AA formulation stimulated 59Fe absorption in iron-deficient male mice. 2.5 µCi of 59Fe in the control (Ctrl) or 4 AA formulation was administered to each mouse by oral, intragastric gavage after a 2-hour fast. One hour later, mice were sacrificed, iron absorption (percent of dose) was calculated (A), and 59Fe activity was determined in the blood (B), duodenum (C), and liver (D). Blood was also taken from the tail vein 30 minutes after gavage, and 59Fe activity was measured (E). Student’s parametric t tests were performed unless variances were significantly different or normality was not achieved, and in that case, a nonparametric t test was performed. n = 10 per group; *P < .05.
The 4 AA formulation stimulated 59Fe absorption in iron-deficient male mice. 2.5 µCi of 59Fe in the control (Ctrl) or 4 AA formulation was administered to each mouse by oral, intragastric gavage after a 2-hour fast. One hour later, mice were sacrificed, iron absorption (percent of dose) was calculated (A), and 59Fe activity was determined in the blood (B), duodenum (C), and liver (D). Blood was also taken from the tail vein 30 minutes after gavage, and 59Fe activity was measured (E). Student’s parametric t tests were performed unless variances were significantly different or normality was not achieved, and in that case, a nonparametric t test was performed. n = 10 per group; *P < .05.
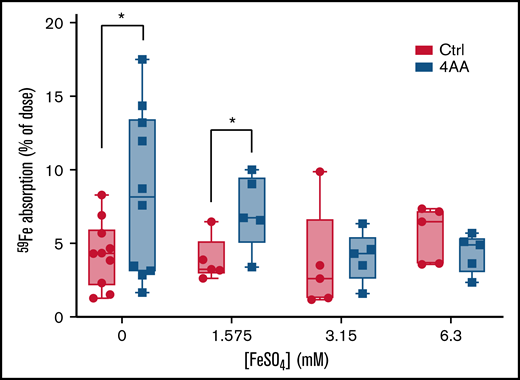
Experiments were thus designed to quantify intestinal iron transport with different doses of iron added to the gavage solution. The concentrations of iron used were chosen based upon previous iron depletion/repletion studies in SW mice (data not shown) and included 1.575, 3.15, and 6.3 mM (and a control group with no cold iron). Outcomes demonstrated that the positive effect of the 4 AAs on 59Fe absorption was again observed when no added iron was included (in the control group) and also when FeSO4 was added at 1.575 mM (Figure 6). At higher iron concentrations, the effect of the 4 AAs was abolished, indicating that there is a threshold of enteral iron in which the 4 AAs exert their influence on iron transport. Overall, these data exemplified the ability of the 4 AAs to increase iron absorption in the presence of supplemental iron, suggesting that this approach could be useful for iron repletion of iron-deficient individuals.
The 4 AAs stimulated iron absorption in iron-deprived male mice in the presence of 1.575 mM FeSO4. 2.5 µCi of 59Fe in the control (Ctrl) or 4 AA formulation, containing 0, 1.575, 3.15, or 6.3 mM FeSO4, was administered to each mouse by oral, intragastric gavage. Mice were sacrificed 1 hour after gavage to determine the percent of dose absorbed. Data were analyzed by Student’s t test at each iron concentration; n = 5 or 10 mice per group; *P < .05.
The 4 AAs stimulated iron absorption in iron-deprived male mice in the presence of 1.575 mM FeSO4. 2.5 µCi of 59Fe in the control (Ctrl) or 4 AA formulation, containing 0, 1.575, 3.15, or 6.3 mM FeSO4, was administered to each mouse by oral, intragastric gavage. Mice were sacrificed 1 hour after gavage to determine the percent of dose absorbed. Data were analyzed by Student’s t test at each iron concentration; n = 5 or 10 mice per group; *P < .05.
4 AAs did not increase 59Fe flux in Ussing chamber experiments with DMT1int/int mice
This investigation has demonstrated that 4 AAs induce trafficking of DMT1 to the enterocyte BBM and enhance iron absorption. Whether DMT1 is solely responsible for increased iron transport, however, is uncertain. Further experiments were thus designed to determine whether the 4 AAs can also stimulate iron transport when intestinal DMT1 is specifically ablated. Ussing chamber flux studies were performed with duodenal epithelial sheets isolated from DMT1int/int mice. Net iron flux (Jnet) was extremely low in all mice, confirming that DMT1 is required for the bulk of intestinal iron transport11,14 (Figure 7A). The 4 AAs did not increase iron flux (as it did in iron-deficient mice) (Figure 6) or conductance (as it did in normal mice) (Figures 1 and 7B). These observations suggest the 4 AAs specifically target DMT1 to enhance iron absorption. Important caveats, however, do exist with this interpretation since DMT1int/int mice were on the 129S6 background and were severely anemic. Additional experimentation is thus required to strengthen this conclusion.
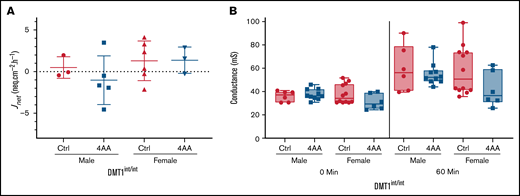
Ablation of intestinal DMT1 abolished the increase in iron flux induced by exposure to the 4 AA formulation. Duodenal epithelial tissues isolated from DMT1 intestine-specific knockout (DMT1int/int) mice were mounted in Ussing chambers and bathed in control or 4 AA formulations. Tissues were paired based on conductance, 15 µCi of 59Fe was added to one side of the chamber, and samples were acquired every 15 minutes from the other side for 1 hour. Jnet was calculated by subtracting Jsm from Jms. No significant difference was observed with respect to 59Fe flux (A) (n = 3-6 per group for Jnet) or conductance (B) (n = 6 to 12 per group) in the presence of the control or 4 AA formulations. Data were analyzed by Student’s t test for each sex and at each time point for conductance.
Ablation of intestinal DMT1 abolished the increase in iron flux induced by exposure to the 4 AA formulation. Duodenal epithelial tissues isolated from DMT1 intestine-specific knockout (DMT1int/int) mice were mounted in Ussing chambers and bathed in control or 4 AA formulations. Tissues were paired based on conductance, 15 µCi of 59Fe was added to one side of the chamber, and samples were acquired every 15 minutes from the other side for 1 hour. Jnet was calculated by subtracting Jsm from Jms. No significant difference was observed with respect to 59Fe flux (A) (n = 3-6 per group for Jnet) or conductance (B) (n = 6 to 12 per group) in the presence of the control or 4 AA formulations. Data were analyzed by Student’s t test for each sex and at each time point for conductance.
Discussion
This investigation has identified 4 AAs that increase the rate of intestinal iron absorption in mice. One pertinent consideration is whether the 4 AAs stimulate iron transport when supplemental iron is included in the transport dose. Since high enteral iron may cause DMT1 to traffic off the BBM,22 we postulated that an iron concentration threshold existed, above which the 4 AAs would be ineffective. Indeed, the 4 AA formulation increased iron absorption when ∼26 µg of iron (ie, 1.5 mM FeSO4 in 300 µL of electrolyte buffer) was included in the gavage dose, but higher amounts of iron abolished this effect. So, how does ∼26 µg of iron in an oral dose for a mouse relate to amounts used for human supplementation? Allometric scaling of drug doses from experimental animals to humans considers body surface area in relation to body mass.23 However, using only mass is a logical approach here since iron does not have a defined half-life in a living animal (unlike a pharmacological compound). So, an adult mouse weighs ∼25 g, and a typical adult human weighs ∼70 kg (or 70 000 g); thus, the average human outweighs the average mouse by 2800-fold (70 000 g/25 g = 2800). Then, 26 µg of iron (the amount in the gavage dose) times 2800 equals 72 800 µg of iron, or 72.8 mg. This amount of iron is on the high end of supplemental doses used to treat IDA in humans. For example, the WHO recommends that 30 to 60 mg of iron be provided daily to prevent anemia in pregnant women,24 while the Centers for Disease Control and Prevention in the United States recommends iron at 30 mg per day during pregnancy.25 In most other cases, lower (or intermittent) iron dosing is effective. In sum, then, the 4 AAs stimulated intestinal iron absorption when supplemental iron was included in the gavage dose in a quantity that extrapolates well to amounts used to treat human ID and IDA.
These observations raise an important question: how do these 4 AAs stimulate intestinal iron transport? A previous investigation found that Gln and Glu increased iron absorption from ligated duodenal loops in anesthetized rats.9 These authors suggested that iron chelation by AAs could enhance absorption, a concept also proposed by others.26 Moreover, an iron-glycine chelate (ferrous bis-glycinate) has been effectively used for human iron supplementation.27,28 Importantly, an iron-AA chelate is probably absorbed differently from inorganic iron, possibly via peptide/AA transporters26 or endocytosis.29 Here, the 4 AAs failed to increase iron flux in duodenal epithelial sheets isolated from mice lacking intestinal DMT1; thus, stimulation of iron transport via the 4 AA formulation probably did not involve chelation. Another plausible mechanism by which AAs may influence iron absorption is via transporter trafficking to the enterocyte BBM, a phenomenon that has been documented by us previously.7,8,12 Supporting this possibility, 1 hour after peroral administration of the 4 AA formulation to live mice, DMT1 protein levels increased in duodenal BBMVs. Higher DMT1 correlated with enhanced iron flux in Ussing chambers and iron absorption in mice, demonstrating physiological significance. Collectively then, these observations suggest that this 4 AA formulation enhances iron absorption via a protein-trafficking mechanism.
One limitation of this work relates to the identification of the 4 AAs since large interanimal variation in DMT1 expression precluded selecting AAs based upon statistical analyses. Although the 4 AAs were effective at increasing DMT1 protein abundance in BBMVs and in stimulating 59Fe absorption in iron-deprived mice, other AAs (alone or in combination) could also be effective. Using the laboratory mouse to model human physiology is another limitation of this study; however, studies in mice have faithfully recapitulated many aspects of iron metabolism in humans.30,31 Future refinement of the 4 AA formulation (eg, by kinetic analysis of each individual AA to optimize concentrations) will precede additional in vivo testing in preclinical rodent models of human iron supplementation.
Acknowledgments
This investigation was funded by grants R01 DK074867 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), and R01 DK109717 from NIDDK and the Office of Dietary Supplements (to J.F.C.).
Authorship
Contribution: R.R.W., S.V., and J.F.C. conceptualized this investigation; R.R.W., Y.Y., X.X., J.K.L., S.Z., J.S.S., and P.E. performed experiments; R.R.W., S.V., B.R.S., and J.F.C. provided intellectual input, designed experiments, and interpreted data; R.R.W. and J.F.C. drafted the manuscript; R.R.W. performed statistical analyses and made the figures; and all authors approved the final, submitted version of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: James F. Collins, Food Science & Human Nutrition Department, University of Florida, FSHN Bldg., #441, 572 Newell Drive, Gainesville, FL 32611; e-mail: jfcollins@ufl.edu.
References
Author notes
Requests for data sharing may be submitted to James F. Collins (jfcollins@ufl.edu).
The full-text version of this article contains a data supplement.