Key Points
Plasma levels of Reg3α were significantly increased in patients with GI-cGVHD compared with those without GI-cGVHD in 2 independent cohorts.
Reg3α is a prognostic biomarker that identifies the likelihood of NRM in patients with GI-cGVHD.
Abstract
Prognostic biomarkers used to identify likelihood of disease progression have not been identified for chronic graft-versus-host disease (cGVHD), the leading cause of late nonrelapse mortality (NRM) in survivors of allogeneic hematopoietic cell transplantation. Gastrointestinal cGVHD (GI-cGVHD) has been particularly challenging to classify. Here, we analyzed 3 proteomics markers (Regenerating islet-derived protein 3-α [Reg3α], C-X-C motif ligand 9 [CXCL9], and Stimulation-2 [ST2]) in 2 independent cohorts of patients with cGVHD totaling 289 patients. Plasma concentrations of Reg3α were significantly increased in patients with GI-cGVHD (P = .0012) compared with those without (P = .01), but plasma concentrations of CXCL9 and ST2 were not. Patients with high Reg3α (≥72 ng/mL) vs low Reg3α had higher NRM (23% vs 11%; P = .015). Because Reg3α has been identified as a lower GI tract marker in acute GVHD, we correlated Reg3α with lower acute-like GI-cGVHD vs classical fibrotic-like esophageal manifestations and found that Reg3α did not differ between the subtypes. No difference was observed between upper GI tract and lower GI tract subtypes. Patients with extremely high Reg3α (≥180 ng/mL) had higher GI scores but not higher scores for the lower GI tract. In a multivariable Cox regression model, patients with high Reg3α were 1.9 times more likely to die without relapse. Our findings demonstrate the utility of Reg3α as a prognostic marker for GI-cGVHD. These data warrant prospective biomarker validation studies.
Introduction
Chronic GVHD (cGVHD) remains a major contributor to morbidity and mortality for survivors of allogeneic hematopoietic cell transplantation (allo-HCT), occurring in up to 50% of patients receiving a T-cell-replete graft.1 Gastrointestinal (GI) involvement is associated with greater risk of nonrelapse mortality (NRM), and patients with severe GI-cGVHD in the lower GI tract are typically treated like patients with acute GVHD (aGVHD).2-4 The National Institutes of Health (NIH) consensus 2005 (updated in 2014) provided the first definition and severity grading for GI manifestations in the context of cGVHD.5 It now requires the presence of diagnostic or distinctive symptoms to separate cGVHD from aGVHD.5 It should be noted that only esophageal stricture or stenosis and histologic changes in mucosa are distinctive criteria of GI-cGVHD, but diarrhea is not.5 Strictly applying the 2014 NIH criteria, respective incidence rates of esophageal, upper GI tract, and lower GI tract involvement are 16%, 20%, and 13% according to a cross-sectional analysis from the cGVHD Consortium.6 These rates have not been confirmed in independent cohorts. Furthermore, longitudinal observational studies revealed a significant number of incorrectly classified patients,7 suggesting that noninvasive, objective stratification of GI-cGVHD patients who will develop worse prognosis is an unmet need. We previously identified chemokine C-X-C motif ligand 9 (CXCL9) and Stimulation-2 (ST2) as biomarkers in cGVHD.8,9 However, their roles in GI-cGVHD have not been explored. Regenerating islet-derived protein 3-α (Reg3α), a c-type lectin expressed in Paneth cells, previously validated as a diagnostic biomarker for GI-aGVHD in the lower GI tract10,11 and as a marker for NRM in combination with ST2 when measured as early as day 7 after HCT,12 has also not been measured in GI-cGVHD. The importance of identifying cGVHD prognostic biomarkers (used to identify the likelihood of disease progression)13 has been outlined in the 2020 NIH consensus because they will allow for preemptive therapies.1,14,15 To address various gaps in our knowlege, we report the incidence of and biomarkers for GI-cGVHD in 2 independent cohorts totaling 289 patients with cGVHD. We show that plasma concentration of Reg3α at the onset of cGVHD is a biomarker for GI-cGVHD and NRM, independent of other factors.
Methods
Plasma samples and patient information were collected after obtaining consent in accordance with institutional review board–approved studies at participating institutions. The study was conducted in accordance with the Declaration of Helsinki. Patients with cGVHD were enrolled at the University of Michigan (UM; n = 111) and Fred Hutchinson Cancer Research Center (FHCRC; n = 178). Patients were separated into those with GI-cGVHD and those without per NIH 2014 diagnostic criteria for cGVHD5 and into esophageal, upper GI tract, and lower GI tract subtypes (Table 1; supplemental Table 1). Samples were obtained at cGVHD diagnosis. NIH and GI scores at sample, and maximum scores and scores for the lower GI tract were evaluated (Table 1; supplemental Table 2).
Enzyme-linked immunosorbent assay (ELISA) kits were purchased from MBL International, RayBiotech, and R&D Systems for Reg3α, CXCL9, and ST2, respectively. Samples (diluted 1:10, 1:20, and 1:50, respectively) and standards were run in duplicate. Absorbance was measured with a SpectraMax M2 microplate reader from Molecular Devices.
Population proportions were compared using the χ2 test. Biomarker concentrations were presented as mean ± standard error of the mean (SEM) and compared using the Mann-Whitney U test. All tests were 2-sided at a significance level of 0.05, and Bonferroni correction was used for multiple comparisons. Cumulative incidences of NRM were analyzed with relapse as a competing risk. Kaplan-Meier survival analysis was used to compute overall survival (OS).
Results and discussion
The UM cohort included 23 patients with GI-cGVHD and 88 without (incidence of 21%). The FHCRC cohort included 63 patients with GI-cGVHD and 115 without (incidence of 35%; Table 1). Reports of incidence of GI-cGVHD are limited in the literature, but they are consistent with a recent study reporting incidence of 31% in a cohort of 567 patients.6 There was no significant difference between patients with GI-cGVHD and those without regarding age, donor type, stem cell source, conditioning regimen, previous aGVHD, progressive GVHD, time to cGVHD diagnosis, time to sample, and NIH score at sample and maximum (Table 1). In evaluable patients, only 3 with GI-cGVHD of the upper GI tract went on to develop GI-cGVHD of the lower GI tract (supplemental Table 2).
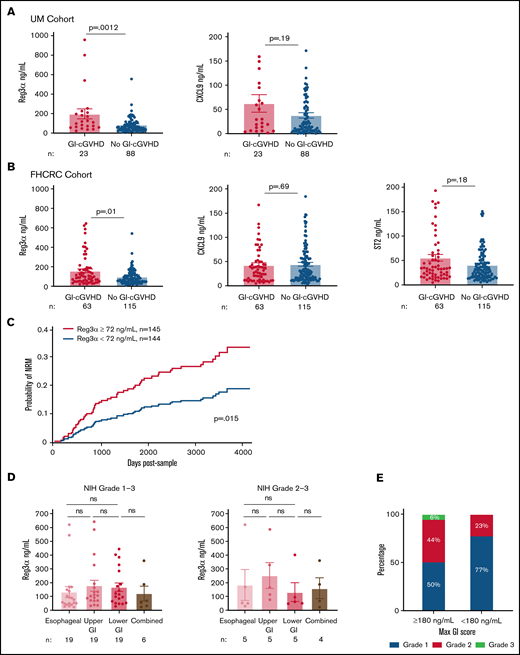
Plasma biomarkers were measured using ELISA. ST2 was measured only in the FHCRC cohort. There was weak correlation between Reg3α, CXCL9, and ST2 concentrations (supplemental Table 3). The mean ± SEM for Reg3α was higher in patients with GI-cGVHD in both UM (P = .0012) and FHCRC (P = .01) cohorts, but means ± SEM for CXCL9 and ST2 were not (Figure 1A-B). These data suggest that a GI-specific protein is a better marker for GI-cGVHD than systemic markers.
Reg3α as a biomarker of GI-cGVHD in two independent cohorts with prognostic significance, irrespective of GI-cGVHD subtype. (A) Plasma concentrations of Reg3α and CXCL9 in patients with (n = 23) and without (n = 88) GI-cGVHD in the UM cohort (n = 111). (B) Plasma concentrations of Reg3α, CXCL9, and ST2 in patients with (n = 63) and without (n = 115) GI-cGVHD in the FHCRC cohort (n = 178). (C) Cohorts combined (n = 289) and dichotomized based on median Reg3α of 72 ng/mL into groups with high levels (≥72 ng/mL; n = 145) and low levels (<72 ng/mL; n = 144). NRM was calculated with relapse as a competing risk from time of sample acquisition (days). (D) Plasma concentrations of Reg3α compared between NIH score of 1 to 3 esophageal (n = 19), upper GI tract (n = 19), lower GI tract (n = 19), and combined (n = 6) GI-cGVHD manifestations in the FHCRC cohort. Plasma concentrations of Reg3α were compared between severe NIH scores of 2 to 3 esophageal (n = 5), upper GI tract (n = 5), lower GI tract (n = 5), and combined (n = 4) GI-cGVHD manifestations in the FHCRC cohort. (E) Proportions of maximum GI score in FHCRC cohort patients with GI-cGVHD divided into extremely high Reg3α levels (≥180 ng/mL; n = 16) and low levels (<180 ng/mL, n = 47). ns, not significant.
Reg3α as a biomarker of GI-cGVHD in two independent cohorts with prognostic significance, irrespective of GI-cGVHD subtype. (A) Plasma concentrations of Reg3α and CXCL9 in patients with (n = 23) and without (n = 88) GI-cGVHD in the UM cohort (n = 111). (B) Plasma concentrations of Reg3α, CXCL9, and ST2 in patients with (n = 63) and without (n = 115) GI-cGVHD in the FHCRC cohort (n = 178). (C) Cohorts combined (n = 289) and dichotomized based on median Reg3α of 72 ng/mL into groups with high levels (≥72 ng/mL; n = 145) and low levels (<72 ng/mL; n = 144). NRM was calculated with relapse as a competing risk from time of sample acquisition (days). (D) Plasma concentrations of Reg3α compared between NIH score of 1 to 3 esophageal (n = 19), upper GI tract (n = 19), lower GI tract (n = 19), and combined (n = 6) GI-cGVHD manifestations in the FHCRC cohort. Plasma concentrations of Reg3α were compared between severe NIH scores of 2 to 3 esophageal (n = 5), upper GI tract (n = 5), lower GI tract (n = 5), and combined (n = 4) GI-cGVHD manifestations in the FHCRC cohort. (E) Proportions of maximum GI score in FHCRC cohort patients with GI-cGVHD divided into extremely high Reg3α levels (≥180 ng/mL; n = 16) and low levels (<180 ng/mL, n = 47). ns, not significant.
NRM was our primary outcome of interest. Because medians in both cohorts were similar, they were combined (characteristics in supplemental Table 4) and dichotomized into high and low Reg3α groups based on the median value of 72 ng/mL. Of note, overall NRM for this combined cohort was 18% (95% confidence interval (CI), 13%-23%) at 2000 days after sample (supplemental Figure 1). Patients with high Reg3α values (≥72 ng/mL) had higher NRM than patients with low Reg3α values (<72 ng/mL) (23% [95% CI, 17%-32%] vs 11% [95% CI, 7%-19%]) at 2000 days after sample (P = .015) (Figure 1C; supplemental Figure 2). OS was not different in patients with GI-cGVHD compared with those without (P = .15), whereas patients with high Reg3α trended toward lower OS than patients with low Reg3α (P = .08; supplemental Figure 3A-C). These data suggest that Reg3α is a mortality predictor and will need to be prospectively validated.
Because Reg3α is a marker for the lower GI tract,10 we hypothesized that high Reg3α will be seen in GI-cGVHD in the lower GI tract rather than fibrotic-like esophageal manifestations. In the FHCRC cohort, all 63 patients with GI-cGVHD had subtypes reported. Distribution was 30% esophageal, 30% upper GI tract, 30% lower GI tract, and 10% combined esophageal and lower GI tract, similar to those for the cGVHD Consortium report (supplemental Table 5).6 We did not find Reg3α differences in mean ± SEM in esophageal, lower GI tract, or combined manifestations irrespective of scores (Figure 1D; supplemental Figure 4). As expected, patients with maximum GI scores of 2 to 3 had higher NRM than those with maximum GI scores of 1 (supplemental Figure 5). Sixteen patients with extremely high Reg3α (≥180 ng/mL) had higher GI scores at sample and maximum, but not higher scores for the lower GI tract (Figure 1E; supplemental Table 6). This suggests that Reg3α was unexpectedly not different between classical esophageal and GI-cGVHD in the lower GI tract. One explanation is that Reg3α is also expressed in the pancreas and skin (https://www.proteinatlas.org).16 Pancreatitis has been previously described in patients with cGVHD.17,18 However, amylase and lipase levels were not available. Reg3α has been reported in psoriatic skin inflammation and correlated with interleukin-17a (IL-17a) production in the same skin lesions.19-21 Therefore, we assessed Reg3α concentrations in esophageal GI-cGVHD patients with skin cGVHD and found increased Reg3α compared with patients without skin cGVHD, although the difference was not significant (supplemental Figure 6). In a multivariable Cox regression model for NRM, high Reg3α (≥72 ng/mL) remained significant independently of the NIH score (hazard ratio, 1.9; P = .02; supplemental Table 7).
We conclude that incidence of GI-cGVHD beyond day 100 after HCT is high (up to 35%), which is consistent with a recent report.6 In contrast, a pediatric population showed high incidence of early GI-cGVHD.7 GI-cGVHD did not correlate with previous aGVHD or progressive GVHD, suggesting minimal overlap between aGVHD and cGVHD in this primarily adult study.22 High Reg3α correlated with GI-cGVHD, but not systemic markers. Reg3α was also associated with higher NRM. Surprisingly, Reg3α, even at extreme concentrations, was not associated with GI-cGVHD of the lower GI tract, possibly because of its association with skin cGVHD.
Altogether, this study identifies Reg3α as a prognostic biomarker for GI-cGVHD, independent of disease localization. A limitation of our study is its retrospective nature, so findings need to be validated in a prospective study with sample collection at the onset of GI-cGVHD. If validated, Reg3α could be used as a noninvasive method to identify patients at increased risk of worse outcomes when they have GI-cGVHD.
Acknowledgments
This study was supported by grants from the National Institutes of Health, National Cancer Institute (R01CA168814 [S.P.], R01CA264921 [S.P.], and R01CA118953 [S.J.L.]) and the National Heart, Lung, and Blood Institute (R21HL139934 [S.P.]).
Authorship
Contribution: B.P.D. analyzed the data and helped write the manuscript; H.L. was the study statistician; A.B. analyzed the data and helped write the manuscript; L.O. contributed to study statistics and collected clinical data; D.C. contributed to discussions of the research and helped write the manuscript; S.J.L. contributed to patient accrual, clinical data collection, quality assurance, and discussions of the research and helped write the manuscript; and S.P. conceived and planned the study design, performed the experiments, interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: S.J.L. received research funding from Amgen, AstraZeneca, Incyte, Kadmon, Novartis, Pfizer, Syndax Pharmaceuticals, and Takeda; received study medication from Johnson & Johnson; and served on the steering committee for Incyte; S.P. holds intellectual property rights on biomarkers and the assay to detect cGVHD. The remaining authors declare no competing financial interests.
Correspondence: Sophie Paczesny, Medical University of South Carolina, Basic Sciences Building, Suite 203, 173 Ashley Ave, MSC 504, Charleston, SC 29425; e-mail: paczesns@musc.edu.
References
Author notes
Requests for data sharing may be submitted to Sophie Paczesny (paczesns@musc.edu).
The full-text version of this article contains a data supplement.