TO THE EDITOR:
Despite high initial response rates, most patients with relapsed/refractory large B-cell lymphoma (LBCL) treated with CD19 chimeric antigen receptor T cells (CAR-T) will progress. Best overall response rates with axicabtagene ciloleucel (axi-cel) and tisagenlecleucel (tisa-cel) are 50% to 80%, only half of which are durable.1-3
Pretreatment factors like lactate dehydrogenase (LDH) and Eastern Cooperative Oncology Group (ECOG) performance status (PS) are associated with outcome after CAR-T2,4,5 and inform upfront patient selection, but have no proven role in postinfusion risk-stratification. Prospective, early identification of patients who will experience transient vs durable CAR-T responses could in the future provide the rationale for targeted combination approaches to counteract CAR-T failure.
Indeed, detection of CAR-T failure prior to frank relapse may improve patient outcomes. Currently, only half of patients with post–CAR-T progression receive further treatment, reflecting the rapid clinical deterioration in this population.6 Furthermore, only 20% to 25% of patients achieve prolonged remission following post–CAR-T therapies. Potentially more patients could be salvaged if CAR-T failure was detected early.
Fluorodeoxyglucose–positron emission tomography (FDG-PET) imaging using the 5-point Deauville score (DS) is the gold-standard assessment for end-of-treatment response in LBCL.7 Interim PET response provides prognostic information in R-CHOP (rituximab, cyclophosphamide, hydroxydaunorubicin doxorubicin, vincristine [oncovin], and prednisone)-treated patients,8-10 and PET-driven treatment strategies are being investigated.11-13 To date, this has not been evaluated in the context of CAR-T.
In this multicenter retrospective analysis, we assessed early Deauville response after CAR-T in patients with LBCL as a potential tool to guide treatment decisions.
We analyzed 171 consecutive patients with relapsed/refractory LBCL treated with licensed CAR-T across 3 UK centers (Freeman Hospital Newcastle, King’s College Hospital London, University College London Hospital) between February 2019 and December 2020 who were evaluable for response at 1 month and had at least 3 months’ follow-up.
CAR-T eligibility was centrally reviewed by the National CAR-T Clinical Panel. CAR-T product choice was at the center’s discretion. Response was assessed locally according to the 5-point DS system.7 We subclassified DS4 to account for postinflammatory changes after bridging radiotherapy (RT). Patients with DS4 uptake confined to the RT field were classified DS4RT. FDG-PET scans were performed at 1 month (median, 28 days; interquartile range [IQR], 27-29), 3 months (median, 91 days; IQR, 86-97), and 6 months (median, 181 days; IQR, 175-187) postinfusion. Data were collected retrospectively from hospital records. PET scans were analyzed using non-point spread function reconstructions.
Transient response was defined as progressive disease (PD) by month 6 after complete response (CR) or partial response (PR) at the 1-month assessment. Ongoing responses at 6 months were classified as durable. Pretreatment factors were compared using Wilcoxon Mann-Whitney/Kruskal Wallis (continuous variables) or χ2/Fisher’s exact tests (discrete variables). Time to PD, progression-free survival (PFS), and overall survival (OS) were analyzed using Kaplan-Meier analysis and Cox regression. Time was measured from the 1-month scan until first event. Time to PD was analyzed using the method of Fine and Gray with nonrelapse mortality as competing event.
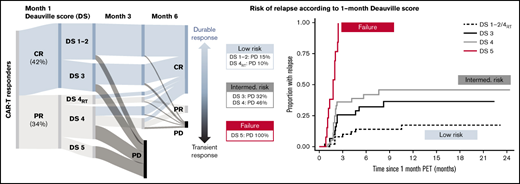
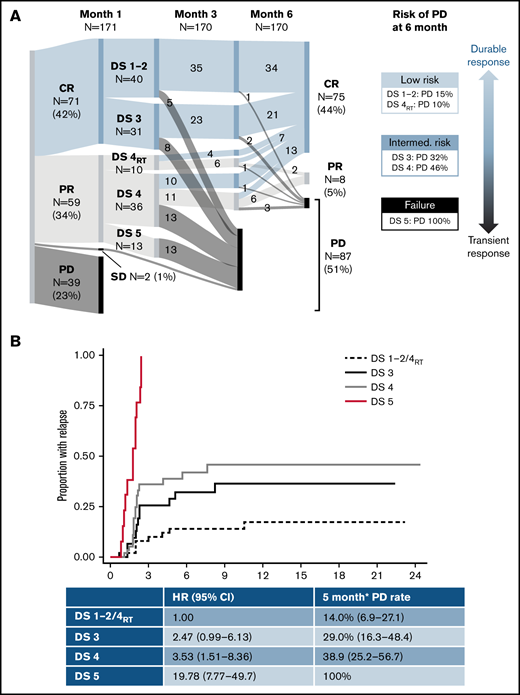
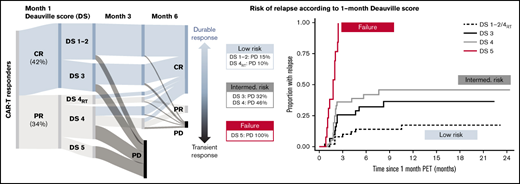
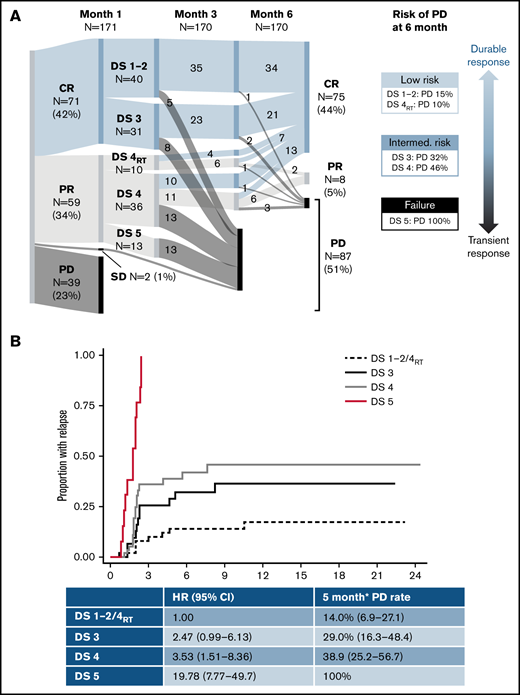
One hundred seventy-one patients were included (130 axi-cel, 41 tisa-cel), with a median follow-up postinfusion of 14.5 months. The median time from approval to infusion was 57 days (IQR, 49-72). One hundred thirty of 171 (76%) patients responded to CAR-T at the 1-month assessment (Figure 1). Forty of 130 (31%) patients had DS1 to 2 response, 31 (24%) patients had DS3 response, 46 (35%) patients had DS4 response, and 13 (10%) patients had PR DS5 response. Forty-six of 129 (36%) responders showed PD at 6 months (transient responders). The study was conducted as a national service evaluation not requiring separate institutional approval. It was conducted in accordance with the Declaration of Helsinki.
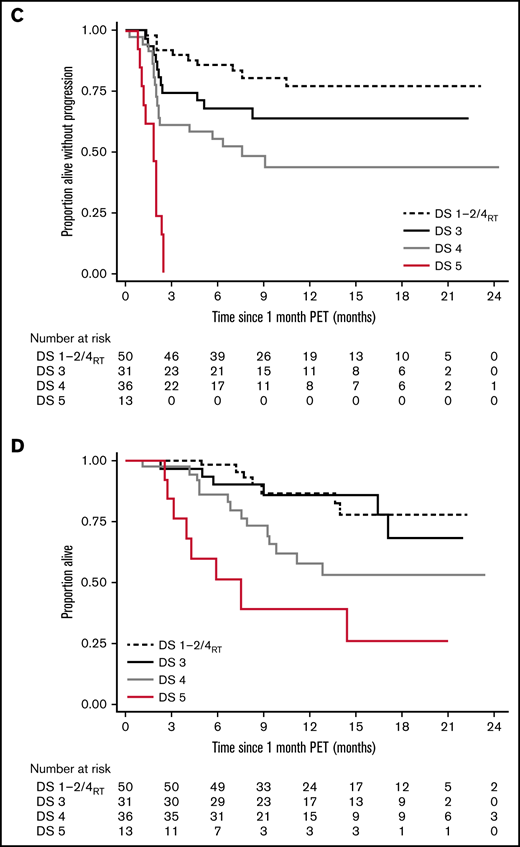
Outcome according to the 1-month DS. Dynamics of response (A), time to relapse (B), PFS (C), and OS (D). SD, stable disease. *6 months postinfusion.
Outcome according to the 1-month DS. Dynamics of response (A), time to relapse (B), PFS (C), and OS (D). SD, stable disease. *6 months postinfusion.
Baseline characteristics are provided in Table 1. Patients with transient vs durable response had higher LDH and C-reactive protein (CRP) preinfusion. Other baseline characteristics (including CAR-T product) did not significantly differ between groups. Deauville categories were significantly associated with durability of response, with a 15% risk of early progression for DS1 to 2, 32% for DS3, 37% for DS4, and 100% for DS5 (Table 1; Figure 1).
Of 46 patients with DS4 response, 15 had received RT bridging therapy within 6 to 8 weeks of the 1-month scan, at which time inflammatory post-RT changes are common. Patients with focal DS4 uptake in the RT field were classified as DS4RT. The DS4RT group behaved similarly to DS1/2 cases with risk of progression at 6 months of 10% vs 46% for the remaining DS4 cases (Table 1; Figure 1). The 1-month Deauville response was not associated with baseline characteristics apart from higher LDH (P = .024) and CRP preinfusion (P = .0018).
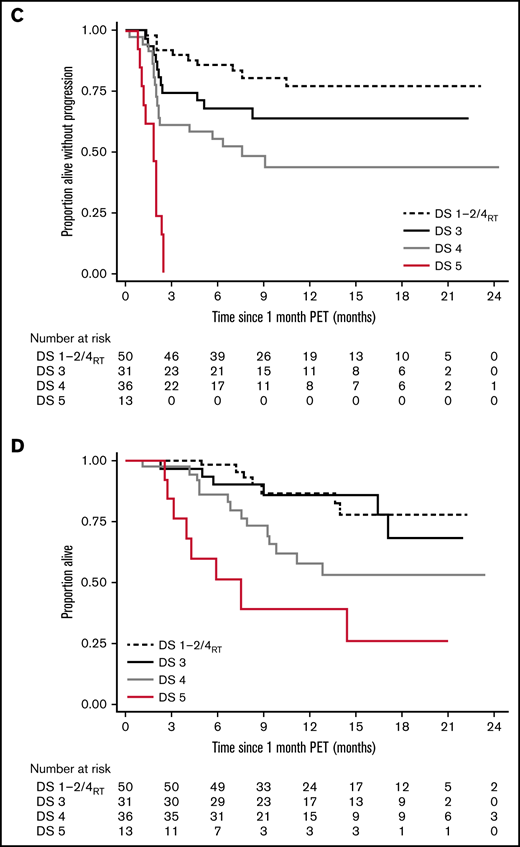
*Rather than having 1 DS cutoff, we considered the predictive power of the 1-month score in 2 ways. Would we want to forgo further treatment in the low-risk group (low false discovery rate when predicting durable responses), and should the high-risk group be considered treatment failures (high specificity)? The DS1 to 2/DS4RT group showed an excellent false discovery rate (14.0%), and the DS5 group showed 100% specificity for predicting transient response (vs 22.5% and 66.3% for a complete response/PR cut point [DS1 to 3 vs 4 to 5]). DS3 and DS4 cases constitute an intermediate-risk group. Time to relapse across groups is shown in Figure 1, with hazard ratio of 3.0 (95% confidence interval [CI], 1.4-6.6) for DS3 to 4 and 19.8 (95% CI, 7.8-49.7) for DS5 vs DS1 to 2/DS4RT. DS groups were the only significant factor for time to relapse in multivariable analysis.
Long-term survival of responding patients significantly differed according to the 1-month DS (Figure 1). Twelve-month PFSs were 77.1% (DS1 to 2/DS4RT), 63.5% (DS3), 43.5% (DS4), and 0% (DS5), and 12-month OSs were 87.1%, 86.2%, 61.7%, and 38.1%, respectively. Patients with SD/PD at 1 month had a 12-month OS rate of 11.5%. The 12-month PFS/OS for the entire cohort was 43.3%/59.7%.
Our results indicate that early FDG-PET response using Deauville criteria may predict the risk of CAR-T failure and be used to guide post–CAR-T management. Although patients achieving early DS1 to 2 remission showed excellent long-term outcomes, patients with DS3 to 4 response had a 31% risk of early relapse, and 46% for DS4 patients when excluding cases with RT-related activity. DS5 response was associated with dismal outcomes and should be regarded as treatment failure.
Response-adapted trial designs of CAR-T in combination with immunomodulatory agents would be an attractive concept, stratified by the 1-month DS. DS1 to 2 patients should be spared additional treatment with potential toxicity, but DS3 to 4 patients with a 30% to 45% risk of early CAR-T failure might benefit from combinatorial approaches. Biomarkers of early response, such as circulating tumor DNA, might help to further delineate insufficient DS4 responses from post–CAR-T inflammation.14
In contrast to DS4, all patients with DS5 at 1 month progressed by month 3. Classifying these patients as “responders” raises unrealistic expectations, and treatment decisions should not be deferred until formal confirmation of PD, particularly if the disease is amenable to RT.
Baseline high-risk factors, including LDH and ECOG PS,2,4,5,15 inform patient selection pre–CAR-T, but by the time patients have undergone treatment and have responded, an individual patient’s risk will have changed. On-treatment biomarkers, including imaging markers of response (eg, DS or disease metabolic volume kinetics16 ), should be incorporated into a dynamic, postinfusion risk model.
Locke et al demonstrated durable responses in axi-cel–treated patients with higher peak CAR-T expansion relative to pretreatment tumor burden, and lower interleukin-6, CRP, and ferritin on the day of infusion.4,17 In our analysis, the strong association of DS response and outcome was independent of preinfusion CRP, but inflammatory markers were not assessed at the 1-month time point.
The difference in PFS by DS category was highly significant and well separated into 4 prognostic groups. The effect on OS was smaller, likely impacted by post–CAR-T treatments.
In conclusion, our results indicate that early FDG-PET DS categories provide a standardized, broadly available tool to predict durable remission after CD19 CAR-T and could inform early post–CAR-T management and response-adapted stratification in clinical trials.
Acknowledgments: The authors thank the patients, their relatives and caregivers, and investigators and staff involved in this analysis.
This work receives funding from the National Institute for Health Research University College London Hospital Biomedical Research Centre (W.T.). I.K. acknowledges funding from the University College London Experimental Cancer Medicine Centre for his post.
Contribution: A.K., C.R., and A.A.K. designed the research, collected the data, analyzed the data, and wrote the manuscript; M.C., M.A.V.M., R.S., M.O., W.T., R.B., V.P., P.E.M.P., and D.Y. contributed to collecting the data and writing the manuscript; and T.M., W.O., S.V., G.S.P., N.M., and I.K. designed the research, collected the data, and wrote the manuscript.
Conflict-of-interest disclosure: A.K. and C.R. have served on advisory boards and received honoraria from Kite/Gilead, Novartis, and BMS. A.A.K. has received honoraria from Kite/Gilead. R.S. and M.O. have served on advisory boards and received honoraria from Kite/Gilead and Novartis. T.M. has served on advisory boards and received honoraria from Kite/Gilead, Novartis, BMS, Janssen, Roche, Servier, Pfizer, and Amgen. W.O. has received honoraria from Roche, Takeda, Pfizer, Servier, Kite/Gilead, MSD, Novartis, Beigene, Astra Zeneca, Syneos, Autolus, Kyowa Kirin, Abbvie, Incyte, and BMS. W.T. has received honoraria and consultancy fees from Celgene BMS, Incyte, and Roche. V.P. has received honoraria from Kite/Gilead. P.E.M.P. has received research funding from Roche, Kite/Gilead, has served on advisory boards for Novartis and Abbvie, has received honoraria from Abbvie, Astra Zeneca, Gilead, Janssen, and Novartis, and has received support for attending meetings from Abbvie and Janssen. D.Y. has served on advisory boards for Gilead and Pfizer and has received support for attending meetings from Servier and Amgen. N.M. has received honoraria from Kite and Novartis. I.K. has received honoraria from Kite/Gilead. The remaining authors declare no competing financial interests.
Correspondence: Andrea Kuhnl, Department of Haematology, King’s College Hospital, Denmark Hill, London SE5 9RS, United Kingdom; e-mail: andrea.kuhnl@nhs.net.
References
Author notes
A.K. and C.R. contributed equally to this study.
N.M. and I.K. contributed equally to this study.
Data can be shared through e-mail request to the corresponding author: andrea.kuhnl@nhs.net.