TO THE EDITOR:
Patients with chronic lymphocytic leukemia (CLL) have an impaired antibody response to COVID-19 vaccination. Here, we evaluated SARS-CoV-2S antibody persistence at 6 months after a two-dose regimen of BNT162b2 COVID-19 vaccine in patients with CLL/small lymphocytic lymphoma (SLL) and healthy controls who had a documented initial response, 2-3 weeks after the second vaccine dose. In a total of 61 patients with CLL/SLL and 39 controls, antibodies were still detectable in 90.2% (n=55) of the patients compared to 100% (n=39) of the controls (adjusted odds ratio=0.11, 95% CI 0.11-1.98; p=0.079). Antibody titers decreased significantly overtime in both patients [from a median of 107.1 U/mL (interquartile range (IQR), 4.6-672.6) to 67.5 U/mL (IQR, 8.0-203.6), p=0.003] and controls [from a median of 983.2 U/mL (IQR, 610.9-1649.0) to 232.9 U/mL (IQR, 157.7-534.1), p<0.001]. Patients on active treatment (n=14) had the lowest seropositivity rate (64.3%) and antibody titers [median=2.8, (IQR, 0.5-17.6)]. Seronegativity occurred in 6 patients; 5 of them continued or initiated treatment during follow-up.In summary, the vast majority of patients with CLL/SLL who initially responded to COVID-19 vaccination remained seropositive at 6 months follow-up. However, antibody titers decreased significantly overtime and low titers or complete antibody loss were associated with active treatment.
Patients with chronic lymphocytic leukemia (CLL) have increased risk for severe coronavirus disease 2019 (COVID-19) as well as mortality.1,2 We and others have reported that patients with CLL/small lymphocytic lymphoma (SLL) have an impaired antibody response to COVID-19 vaccination.3,4 Understanding the long-term persistence of SARS-CoV-2 antibodies after vaccination is highly important to ensure an appropriate vaccination strategy. COVID-19 messenger RNA (mRNA)-based vaccination in healthy subjects has been reported to elicit antibody response that persists for at least 6 months after the second vaccine dose5 ; however, the durability of humoral response in patients with CLL/SLL is still unknown. Here, we report an updated analysis of our primary cohort that now focuses on antibody persistence after a 2-dose regimen of BNT162b2 COVID-19 vaccine in patients with CLL/SLL.
This prospective study was conducted in the framework of the European Research Initiative on CLL. The study was approved by the institutional review board and is registered in ClinicalTrials.gov (no. NCT04746092). All subjects provided informed consent. Eligibility criteria for the study included diagnosis of CLL/SLL according to the International Workshop on Chronic Lymphocytic Leukemia criteria,6 age 18 years or older, with no known history of SARS-CoV-2 infection. The study has also included healthy volunteers that served as a control group. Patients and healthy controls who participated in the primary cohort3 and achieved a serologic response, 2 to 3 weeks after the second vaccine dose, were reevaluated for antibody persistence at 6 months after vaccination. Serum samples were analyzed by the Elecsys Anti-SARS-CoV-2S assay (Roche Diagnostics; supplementary 1). For statistics, see supplementary 2.
The primary cohort included a total of 167 patients with CLL/SLL and 52 healthy subjects. As previously reported,3 66 of 167 patients (39.5%) and all 52 healthy controls obtained a serologic response, when measured at 2 to 3 weeks after the second vaccine dose. Among the responders, 61 patients with CLL/SLL and 39 healthy controls were evaluable for SARS-CoV-2 antibody testing 6 months after the second vaccine dose (patient baseline demographics and disease characteristics are summarized in Table 1). The median time from the second vaccine dose to the second serology test was 171.0 days (interquartile range [IQR], 168.0, 178.5).
Patient baseline demographic and disease characteristics
| Parameter . | Patients with CLL (n = 61) . |
|---|---|
| Age at first serology test, median (IQR), y | 69.4 (59.9-74.6) |
| ≤65, n (%) | 26 (42.6) |
| Males, n (%) | 35 (57.4) |
| Binet stage*, n (%) | |
| A | 27 (81.8) |
| B | 5 (15.2) |
| C | 1 (3.0) |
| IGHV mutational status, n (%) | |
| Mutated | 27 (65.9) |
| Unmutated | 14 (34.1) |
| FISH, n (%) | |
| del(13q) | 13 (33.3) |
| No aberration | 8 (20.5) |
| trisomy12 | 6 (15.4) |
| del(11q) | 11 (28.2) |
| del(17p) | 1 (2.6) |
| β-2-microglobulin | |
| ≤3.5 mg/L | 42 (87.5) |
| >3.5 mg/L | 6 (12.5) |
| Disease/treatment status, n (%) | |
| Treatment-naive | 28 (45.9) |
| On-therapy | 14 (23.0) |
| Previously treated | 19 (31.2) |
| Protocols of currently treated, n (%) | |
| Bruton's tyrosine kinase inhibitors | 9 (64.3) |
| Venetoclax ± anti-CD20 antibody | 5 (35.7) |
| Laboratory parameters, median (IQR) | |
| Absolute lymphocyte count, (×109/L) | 7.6 (2.15-32.5) |
| β-2-microglobulin, mg/L | 2.1 (1.8-3.0) |
| Immunoglobulin G, mg/dL | 969.0 (647.0-1111.0) |
| Immunoglobulin M, mg/dL | 51.5 (28.8-72.5) |
| Immunoglobulin A, mg/dL | 125.0 (66.3-172.3) |
| Parameter . | Patients with CLL (n = 61) . |
|---|---|
| Age at first serology test, median (IQR), y | 69.4 (59.9-74.6) |
| ≤65, n (%) | 26 (42.6) |
| Males, n (%) | 35 (57.4) |
| Binet stage*, n (%) | |
| A | 27 (81.8) |
| B | 5 (15.2) |
| C | 1 (3.0) |
| IGHV mutational status, n (%) | |
| Mutated | 27 (65.9) |
| Unmutated | 14 (34.1) |
| FISH, n (%) | |
| del(13q) | 13 (33.3) |
| No aberration | 8 (20.5) |
| trisomy12 | 6 (15.4) |
| del(11q) | 11 (28.2) |
| del(17p) | 1 (2.6) |
| β-2-microglobulin | |
| ≤3.5 mg/L | 42 (87.5) |
| >3.5 mg/L | 6 (12.5) |
| Disease/treatment status, n (%) | |
| Treatment-naive | 28 (45.9) |
| On-therapy | 14 (23.0) |
| Previously treated | 19 (31.2) |
| Protocols of currently treated, n (%) | |
| Bruton's tyrosine kinase inhibitors | 9 (64.3) |
| Venetoclax ± anti-CD20 antibody | 5 (35.7) |
| Laboratory parameters, median (IQR) | |
| Absolute lymphocyte count, (×109/L) | 7.6 (2.15-32.5) |
| β-2-microglobulin, mg/L | 2.1 (1.8-3.0) |
| Immunoglobulin G, mg/dL | 969.0 (647.0-1111.0) |
| Immunoglobulin M, mg/dL | 51.5 (28.8-72.5) |
| Immunoglobulin A, mg/dL | 125.0 (66.3-172.3) |
Abbreviations: IQR, interquartile range; FISH, fluorescence in situ hybridization; IGHV, immunoglobulin heavy chain.
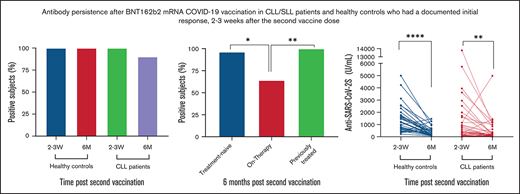
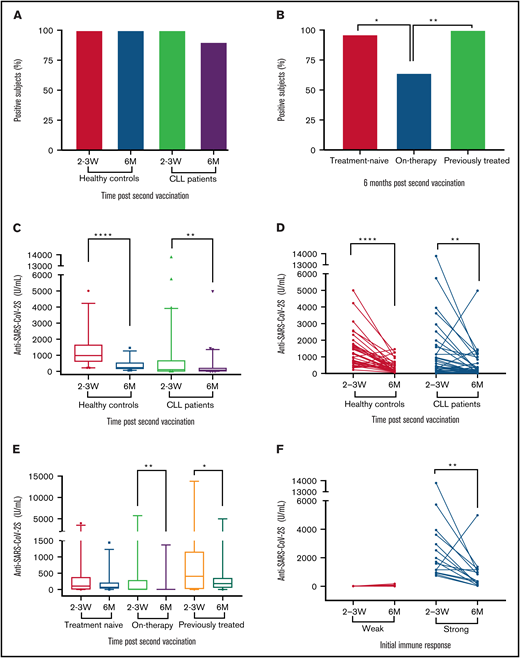
Six months after the second vaccine dose, serum anti SARS-CoV-2s antibodies were still detectable in 90.2% (n = 55) of the patients with CLL compared with 100% (n = 39) of the controls (adjusted odds ratio = 0.11; 95% confidence interval, 0.11-1.98; P = .079; Figure 1A; supplemental Table 1). The seropositivity rate was lowest in patients on active treatment, and significantly inferior in these patients compared with treatment naïve and previously treated patients (P = .002; Figure 1B). A complete loss of antibody titer occurred in 6 patients; 5 of them continued or initiated treatment during the follow-up period (3 treated with venetoclax plus obinutuzumab and 2 with Bruton’s tyrosine kinase inhibitors). Only 3 patients were treated with obinutuzumab during the follow-up peroid, and all became seronegative.
Six-month antibody persistence after BNT162b2 mRNA COVID-19 vaccination in patients with CLL/SLL. (A) Response rate among CLL patients (n = 61) and healthy controls (n = 39, P = .079). (B) Response rate by current status of treatment , a pairwise comparisons: treatment-naïve (n = 28) vs on-treatment (n = 14, P = .018), on therapy vs previously treated (n=19, P = .006), treatment-naïve vs previously-treated (P = .665). (C,D) Anti-SARS-CoV-2S titers (U/mL) in patients with CLL/SLL (n = 61) and healthy controls (n = 39), at 2 to 3 weeks and 6 months after second vaccination. (E) Anti-SARS-CoV-2S titers (U/mL) in patients with CLL/SLL according to disease status, at 2 to 3 weeks and 6 months after second vaccination (treatment-naïve, n = 28; on-treatment, n = 14; previously treated, n = 19). (F) Anti-SARS-CoV-2S titers (U/mL) dynamics over time in patients with CLL/SLL dichotomized to weak (shown are anti SARS-CoV-2S titers of patients who achieved titer levels within the lowest quartile of titer levels) and strong (shown are anti SARS-CoV-2S titers of patients who achieved titer levels within the upper quartile of titer levels) initial antibody response, 2 to 3 weeks after second vaccine dose.
Six-month antibody persistence after BNT162b2 mRNA COVID-19 vaccination in patients with CLL/SLL. (A) Response rate among CLL patients (n = 61) and healthy controls (n = 39, P = .079). (B) Response rate by current status of treatment , a pairwise comparisons: treatment-naïve (n = 28) vs on-treatment (n = 14, P = .018), on therapy vs previously treated (n=19, P = .006), treatment-naïve vs previously-treated (P = .665). (C,D) Anti-SARS-CoV-2S titers (U/mL) in patients with CLL/SLL (n = 61) and healthy controls (n = 39), at 2 to 3 weeks and 6 months after second vaccination. (E) Anti-SARS-CoV-2S titers (U/mL) in patients with CLL/SLL according to disease status, at 2 to 3 weeks and 6 months after second vaccination (treatment-naïve, n = 28; on-treatment, n = 14; previously treated, n = 19). (F) Anti-SARS-CoV-2S titers (U/mL) dynamics over time in patients with CLL/SLL dichotomized to weak (shown are anti SARS-CoV-2S titers of patients who achieved titer levels within the lowest quartile of titer levels) and strong (shown are anti SARS-CoV-2S titers of patients who achieved titer levels within the upper quartile of titer levels) initial antibody response, 2 to 3 weeks after second vaccine dose.
Antibody titers decreased significantly overtime in both patients [from a median of 107.1 U/mL (IQR, 4.6, 672.6) to 67.5 U/mL (IQR, 8.0-203.6), P = .003] and controls [from a median of 983.2 U/mL (IQR, 610.9-1649.0]) to 232.9 U/mL (IQR, 157.7-534.1), P < .001 (Figure 1C-D; supplemental Table 2)]. Overall, at 6 months after the second vaccine dose, antibody titers were lower in patients compared with controls (P < .001) and decreased by a median of 58.2% (IQR, -80.7, -23.9) in patients with CLL and by a median of 77.1% [IQR, -83.0, -64.1 in controls (P = .012)]. Patients on active treatment had the lowest antibody titers at 6 months postvaccination [median 2.8 U/mL, (IQR, 0.5,17.6), Figure 1E; supplemental Tables 3 and 4]. Patients who lost their initial humoral response were those who already had very low antibody levels when measured 2 to 3 weeks after the second vaccine dose [median 1.4 U/mL (IQR, 1.2, 2.2)]. Antibody level kinetics disclosed an over-time decline in antibody titers in most patient subgroups, which was much more pronounced in those with a strong initial immune response (Figure 1F; supplemental Table 4). During the 6-month follow-up of the primary cohort, symptomatic COVID-19 was diagnosed in only 1 seronegative patient and in none of the seropositive subjects.
In this report, we evaluated the persistence of SARS-CoV-2 antibodies after the second BNT162b2 mRNA COVID-19 vaccine dose. In both patients with CLL/SLL and healthy subjects, antibody titers declined significantly over time. However, in most patients and all controls, antibodies were still detectable at 6 months post-vaccination. We found that at 6 months, antibody titers were lower in patients with CLL/SLL compared with the controls and low titers were particularly associated with active treatment. The 6 patients who became seronegative had a poor initial serologic response to the vaccine and most of them either continued or initiated anti-CLL treatments during the post-vaccination period. We also show that in patients who had a robust initial antibody response after a second COVID-19 vaccine dose (patients that were altogether younger and with favorable CLL characteristics), the degree of antibody level decay was more marked than in those with a weak initial response. It was recently reported7 that among fully vaccinated health care workers, the occurrence of breakthrough infections with SARS-CoV-2 correlated with lower levels of both peri-infection neutralizing antibodies and anti spike antibodies (compared with matched uninfected controls). Yet, the peak of the neutralizing and antispike antibodies within the first month after the second vaccine dose was more strongly associated with the risk of breakthrough infections.7
In summary, in the vast majority of patients with CLL who initially responded to a 2-dose regimen of BNT162b2 COVID-19 vaccine, the antibody response was maintained at the 6-month follow-up. However, antibody titers decreased significantly overtime and complete loss of response was associated with active treatment. Given that patients with CLL/SLL are at high risk for severe COVID-19 disease and that SARS-CoV-2 antibody levels reduce over time, it is reasonable to administer a COVID-19 vaccine booster to CLL/SLL patients, 6 months after the second vaccine dose.
Acknowledgments: The authors thank the clinical study coordinators and nurses at the hematology department in the Tel-Aviv Sourasky Medical Centre.
Contribution: Y.H. and I.A. initiated the trial, designed the study analyzed data, and wrote the paper; G.S. and M.M.M. performed the serological testing; Y.B. collected the data; S.L. and T.Z. analyzed the data; L.S. designed the study; C.P. wrote the paper; and P.G. designed the study and wrote the paper.
Conflict-of-interest disclosure: Y.H. reports honoraria from AbbVie, Janssen, Astra-Zeneca, Medision, and Roche outside the submitted work. I.A. reports speaker's bureau for Gilead, Novartis, AbbeVie, and Janssen and served as a consultant for Janssen, MSD, AbbVie, Novartis, and Roche outside the submitted work. E.J. reports advisory board work for AstraZeneca and Epizyme. L.S. reports honoraria from AbbVie, Astra-Zeneca, and Janssen. P.G. reports grants and personal fees from AbbVie, AstrZeneca, Gilead, Janssen, and Sunesis; personal fees from AbbVie, AstraZeneca, Adaptive, Arqule/MSD, BeiGene, Celgene/Juno/BMS, Janssen, Lilly/Loxo, and Roche outside the submitted work. The remaining authors declare no competing financial interests.
For data sharing, contact the corresponding author: yairh@tlvmc.gov.il.
Correspondence: Yair Herishanu, Department of Hematology Tel Aviv Sourasky Medical Center, 6 Weizmann St, Tel-Aviv, Israel 64239; e-mail: yairh@tasmc.health.gov.il.
References
Author notes
Y.H. and I.A. contributed equally to this study.
The full-text version of this article contains a data supplement.