Key Points
RT is a highly effective and safe treatment of GML with excellent overall survival and very rare acute or late treatment-related toxicities.
Spontaneous resolution of pathologic abnormalities with low lymphoma-related mortality may allow less stringent endoscopic follow-up.
Abstract
Treatment options for Helicobacter pylori–independent gastric mucosa-associated lymphoid tissue (MALT) lymphoma (GML) include surgery, immunotherapy, chemotherapy, and radiation therapy (RT). The purpose of this study was to investigate the efficacy and safety of RT and routine endoscopic surveillance, hypothesizing that most patients are curable with RT alone. We queried a single institution database at a tertiary referral cancer center for patients with H pylori–independent GML treated with RT between 1991 and 2017. Response was assessed by follow-up endoscopies (EGDs) starting 10 to 12 weeks post-RT. Computed tomography scans were also part of the follow-up program, and positron emission tomography was added when clinically appropriate. We identified 178 patients (median age, 63 years; range, 25-89 years); 86% had stage I disease, 7% had stage II disease, and 7% had stage IV disease. Median RT dose was 3000 cGy over 20 fractions. Ninety-five percent of patients exhibited complete pathologic response on posttreatment EGD. Two patients experienced grade 3 toxicity, and 2 patients experienced in-field secondary malignancies. Over a median follow-up of 6.2 years, 9.6% experienced local failures, and 11.8% developed distant sites of disease. Five-year and 10-year overall survival were 94% and 79%, respectively, from last date of RT. RT is a highly effective and safe treatment for GML with excellent overall survival and very rare acute or late treatment-related toxicities. Favorable outcomes from this large retrospective sample of patients provide credible and compelling support for RT as standard of care for H pylori–independent GML.
Introduction
Gastric mucosa-associated lymphoid tissue (MALT) lymphoma (GML) is a low-grade B-cell lymphoma and is the most common lymphoid neoplasm arising in mucosa.1 Despite the decline in gastric cancers, the incidence of GML has been increasing and may be influenced by advances in endoscopic evaluation.2,3 Most patients with GML present with early-stage disease and active infection with Helicobacter pylori. H pylori induces a reactive immune response that leads to formation of lymphoid follicles that are normally absent in the stomach mucosa. It is believed that GML arises from this background and is sustained by the presence of H pylori. Eradicating H pylori infection removes the microenvironmental stimuli supporting tumor growth and is an effective treatment in 60% to 80% of patients.4,5
In some cases, GML persists despite H pylori eradication. In these cases of H pylori–independent GML, treatment options include surgery, immunotherapy, chemotherapy, and radiation therapy (RT).6 Partial or total gastrectomy, once regarded as the standard of care, is associated with significant morbidity is used only rarely as salvage treatment.7 Current standard of care is radiation alone as definitive treatment, which was first described by our group in the 1990s.8
Involved-field RT was initially chosen as an appropriate strategy for GML because of the tendency of GML to remain localized for long periods of time. We reported the efficacy and safety of radiation in a cohort of 17 patients with stage I-II2 low-grade lymphoma of the stomach without evidence of H pylori infection.8 With 27 months of median follow-up, event-free survival was 100%.
Since that study, several series have reported excellent long-term outcomes with the use of radiation for GML, and RT has been accepted as standard of care.9-12 Given the indolent course of disease progression, as well as changes in radiation technology and variation with histologic grading of treatment response,13 we sought to update our experience of treating H pylori–independent GML with radiation at a single tertiary referral cancer center. The measurable outcomes of this study included overall survival (OS), cause-specific survival, cumulative incidence of local and distant disease failures, radiation-related toxicities, and secondary malignancies. Serial endoscopic biopsies reviewed by hematopathology experts provided robust data on local control for long-term follow-up. The results of this study, the largest reported to date, are important for guiding the development of new studies to further reduce the morbidity of GML therapy.
Patients and methods
Institutional review board approval
The study was approved by the Memorial Sloan Kettering Cancer Center’s Institutional Review Board (16-1020) and was conducted in accordance with the Declaration of Helsinki.
Patient selection
From November 1991 to March 2017, 178 patients were diagnosed with GML and subsequently treated with radiation to the stomach in the Department of Radiation Oncology at Memorial Sloan Kettering Cancer Center. Prior to treatment, pathology was confirmed by hematopathology experts, and patients underwent radiologic staging with computed tomography (CT) scan, positron emission tomography scan, endoscopic ultrasound, or a combination of these techniques. Demographic, clinical, and treatment details and follow-up data were abstracted via chart review (paper and electronic) and entered into a departmental database.
Treatment and follow-up
All patients received involved-site radiotherapy to the stomach according to previously reported techniques.8,14 The clinical treatment volume included the stomach and first part of the duodenum. Perigastric lymph nodes and other parts of the duodenum were also included in clinical treatment volume if involved with disease. Response was assessed by follow-up endoscopies (EGDs) starting 10 to 12 weeks following RT and continuing in increasing intervals, ranging from 6 to 24 months, throughout the follow-up period. CT scans were also part of the follow-up program; positron emission tomography was added when clinically appropriate. Endoscopic biopsies were reviewed by hematopathology experts, and response was based on Wotherspoon criteria: score 1-2, remission; score 3-4, atypical/suspicious; and score 5, resistant/relapsed lymphoma.15 Radiation fields were reviewed to determine whether additional malignancies occurred within the radiation fields. The last date of database query was in June 2019, and median follow-up time was 6.5 years (range, 1.2-22.8).
Statistical analysis
Kaplan-Meier and cumulative incidence curves were generated to examine the survival and cumulative incidence experiences of patients. Univariable Cox proportional-hazards regression was used to analyze OS and progression-free survival (PFS). Disease failure and local failure were analyzed using Fine and Gray competing risks regression.16 All end points were calculated from the date of last RT. For PFS, events of interest included death from any cause, local failures, or distant disease progression. Local failures were defined as GML relapse, refractory GML, or transformation to diffuse large B-cell lymphoma (DLBCL). Distant failures included MALT or DLBCL diagnosis outside of the stomach. A significance level of 0.05 was used throughout. All statistical computations were performed and all output was generated using SAS Software 9.4 (SAS Institute, Cary, NC).
Results
Patient and treatment characteristics
A summary of patient demographic and clinical characteristics is provided in Table 1. Of the 178 patients identified, 56% were female. Median age at diagnosis was 63 years (range, 25-89). Stage I disease was present in 153 of 178 patients (86%), stage II disease was present in 13 patients (7%), and stage IV disease was found in 12 patients (7%). Median RT dose was 3000 cGy (range, 2250-4350), given over a median of 20 fractions (150 cGy per fraction).
All 178 patients had GML reconfirmed by hematopathology experts prior to RT. Median time from initial GML diagnosis to RT initiation was 4.5 months. Latency between diagnosis and treatment ranged from 3 weeks, for a patient who presented with gastrointestinal bleeding, to 14 years, for a patient who initially elected treatment with rituximab and then proceeded with radiation after subsequent EGDs demonstrated persistent disease.
Over the span of 26 years, treatment techniques evolved with improved technology. Initially, patients were treated with conventional anterior posterior fields (20 patients in this series). Treatments were then refined to 3-dimensional fields to limit radiation dose to kidneys and other radiation-sensitive structures. Beginning in 2004, all patients were treated with image guidance RT (IMRT), as detailed in the field and dose guidelines reported by the International Lymphoma Radiation Oncology Group,14 with 4-dimensional CT planning and a small volume of oral contrast. Patients were always simulated and treated with an empty stomach after a fast of ≥4 hours.
Thirty-five of 178 patients (20%) were initially diagnosed with H pylori–positive GML. These patients were treated with antibiotics and subsequently underwent EGD to assess disease response. For these patients with biopsy-proven antibiotic-refractory GML, median time from diagnosis to RT was 10.2 months. For patients who presented with H pylori–negative disease, median time from diagnosis to RT was 4.1 months. By the start of RT, 174 of 178 patients (98%) were confirmed to be negative for H pylori on biopsy. For the 4 patients with unknown H pylori status, 2 patients were treated because of gastric bleeding, and 2 patients had previously undergone antibiotic therapy.
Survival event analysis
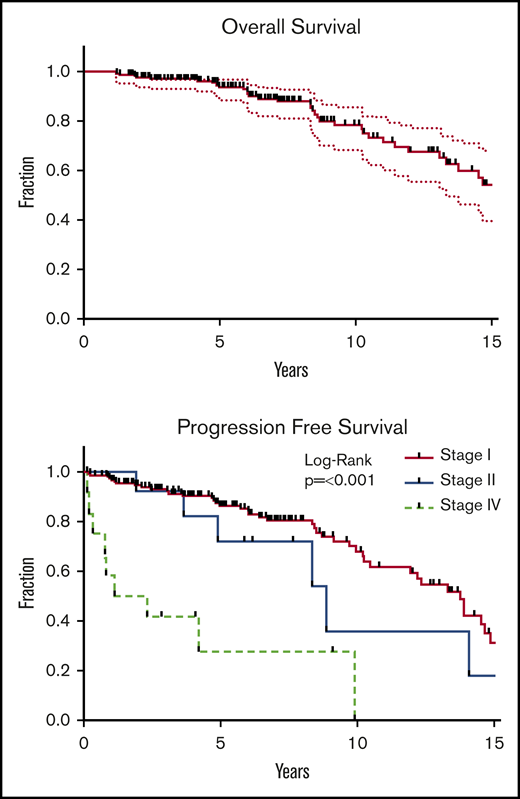
Over a median follow-up period of 6.5 years, 396 patients died. Survivors were followed for a median of 6.2 years (range, 1.6-22.8). Five-year and 10-year OS estimates were 94% and 79%, respectively (Figure 1). Median OS was 16.5 years.
OS and PFS. Kaplan-Meier analysis of OS (upper panel) and PFS (lower panel). OS was defined as survival from all causes and was calculated from date of last RT. End points for PFS included death from any cause, local failure, and distant disease failure. Survivors were followed for a median of 6.2 years (range, 1.6-22.8). Median OS was 16.5 years. Five-year and 10-year OS estimates were 94% and 79%, respectively. Presenting stage was highly significant for PFS (P < .001), local failure (P < .001), and distant failure (P = .003).
OS and PFS. Kaplan-Meier analysis of OS (upper panel) and PFS (lower panel). OS was defined as survival from all causes and was calculated from date of last RT. End points for PFS included death from any cause, local failure, and distant disease failure. Survivors were followed for a median of 6.2 years (range, 1.6-22.8). Median OS was 16.5 years. Five-year and 10-year OS estimates were 94% and 79%, respectively. Presenting stage was highly significant for PFS (P < .001), local failure (P < .001), and distant failure (P = .003).
For the 166 patients who were treated for early-stage disease, median PFS was 13.8 years (Figure 1). Events included 36 deaths and 38 treatment failures (12 local failures and 16 distant failures). All patients presenting with distant metastases (n = 16) were controlled locally. Two patients had lymphoma-related deaths. One patient found to have DLBCL on posttreatment EGD died 23 months following RT. Another patient was found to have lymphoma of the prostate 2 years following gastric radiation; he was treated with chemotherapy and subsequently relapsed in multiple sites, ultimately passing away from neutropenic fever.
Presenting stage was highly significant for PFS (P < .001), with a hazard ratio (HR) of 1.7 for stage II disease (95% confidence interval [CI], 0.8-3.8) and an HR of 7.5 for stage IV disease (95% CI, 3.5-15.8). Presenting stage was also significant for local failure (P < .001) and distant failure (P = .003) (Figure 1). Patients who presented with Lugano stage I, II, or IV disease had a median PFS 13.8, 8.9, or 1.7 years, respectively. Five-year and 10-year rates of OS and PFS, as well as the cumulative incidence of local and distant failures, are shown in Table 2. On univariable analysis, age was associated with PFS, but not gender, H pylori status, or latency between diagnosis and treatment.
Age at diagnosis was the only characteristic that had a statistically significant association with OS (P < .001). OS was not associated with disease failure. For the 17 patients who experienced local failures, median OS was 16.2 years compared with 22.9 years for the 21 patients who experienced distant failures and 16.5 years for the 141 patients who did not have any evidence of disease from GML.
Endoscopic pathologic abnormalities
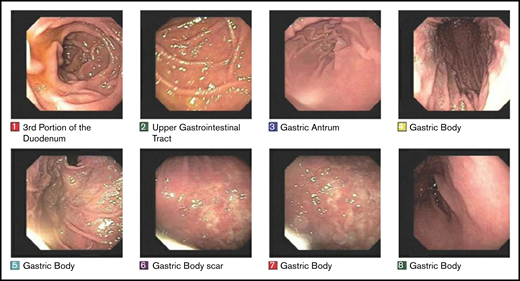
Treatment response to radiotherapy was assessed at 3 months by follow-up EGDs with multiple biopsies (Figure 2). Among the 166 patients who were treated for early-stage GML, posttreatment EGD with biopsy was performed in 160 patients, of whom 152 (95%) demonstrated a complete pathologic response (Wotherspoon 1-2) on the first posttreatment biopsy. Patients were serially followed by EGD biopsies in increasing intervals of 3 to 24 months.
EGD images documenting pathologic complete response. EGD images from patient 6, whose experienced spontaneous normalization without salvage treatment.
EGD images documenting pathologic complete response. EGD images from patient 6, whose experienced spontaneous normalization without salvage treatment.
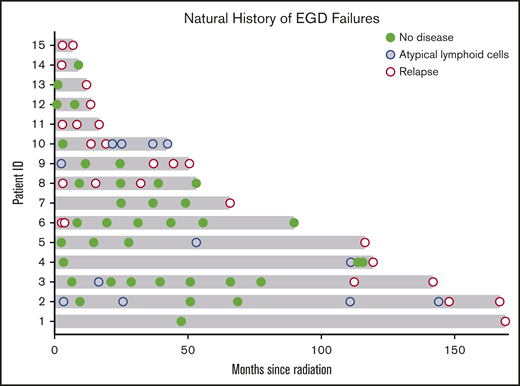
Over this extended follow-up period, 15 of 160 patients (9%) were found to have residual disease on EGD posttreatment biopsy (Wotherspoon 5; Figure 3). In addition, atypical lymphoid cells, not diagnostic of lymphoma (Wotherspoon 3-4), were described in 15 of 160 patients (9%). Six of the 15 patients (40%) with residual lymphoid cells subsequently progressed to local failure. Three of 20 patients (15%) with posttreatment residual disease experienced spontaneous resolution of their pathologic abnormalities on subsequent biopsy and, thus, were considered free of disease. The median interval from last radiation treatment to spontaneously resolved biopsy was 9 months (range, 8 months-3 years).
Natural history of EGD abnormalities. Fifteen patients who presented with early-stage GML exhibited residual GML on posttreatment EGD biopsy. Pathology review reported no evidence of disease (green circle), atypical lymphoid cells (blue circle), or residual GML (red open circle). Five patients with atypical lymphoid cells subsequently progressed to local failure (patients 2, 3, 4, 5, and 9). Three patients (6, 8, and 14) had pathologic EGD failures that self-resolved with observation alone.
Natural history of EGD abnormalities. Fifteen patients who presented with early-stage GML exhibited residual GML on posttreatment EGD biopsy. Pathology review reported no evidence of disease (green circle), atypical lymphoid cells (blue circle), or residual GML (red open circle). Five patients with atypical lymphoid cells subsequently progressed to local failure (patients 2, 3, 4, 5, and 9). Three patients (6, 8, and 14) had pathologic EGD failures that self-resolved with observation alone.
Local failures
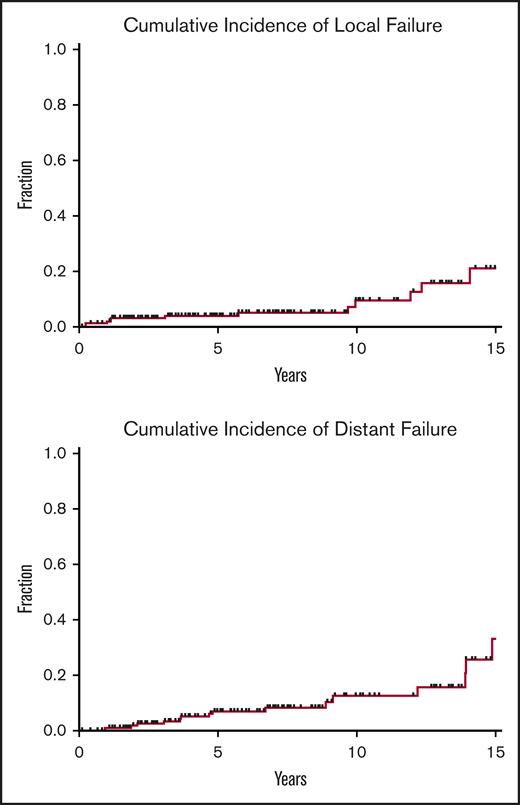
Over a median follow-up of 6.2 years from the end of RT (range, 19.7 months-23 years), 12 of 166 patients (7%) treated for early-stage disease experienced local failures. Five-year and 10-year local failure rates, using death as a competing risk, were 3.9% and 8.3%, respectively (Figure 4). Median time to local disease failure was 4.4 years (range, 3 months-14 years). One patient who developed DLBCL of the stomach 1 year after RT was treated with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) chemotherapy and died of disease a year later. The remaining local relapses were associated with refractory GML on EGD biopsy. Salvage therapies included rituximab, additional radiotherapy, and rituximab (R)-CHOP.
Local and distant disease failures. Cumulative incidence of local and distant failures for patients treated for early-stage GML (N = 166), using death as a competing risk. Five-year and 10-year local failure rates were 3.9% and 8.3%, respectively, whereas 5-year and 10-year distant failure rates were 6.9% and 11%, respectively.
Local and distant disease failures. Cumulative incidence of local and distant failures for patients treated for early-stage GML (N = 166), using death as a competing risk. Five-year and 10-year local failure rates were 3.9% and 8.3%, respectively, whereas 5-year and 10-year distant failure rates were 6.9% and 11%, respectively.
Distant failures
Among patients treated for early-stage disease, 16 of 166 patients (10%) developed distant sites of disease. Five-year and 10-year distant failure rates were 6.9% and 11%, respectively (Figure 4). Median time to distant disease failure was 4.7 years (range, 11 months-15 years). Four of these 16 patients (25%) presented with transformation to DLBCL at distant sites, including colon and retroperitoneum (2 patients each). Median time to DLBCL transformation was 3.6 years (range, 11 months-4.7 years). Despite distant relapse, all 16 patients were found to be free of local disease on posttreatment EGD biopsy. Subsequent treatments included chemotherapy, additional radiation, rituximab monotherapy, salvage surgery, and observation.
Treatment toxicities
Treatment-related toxicities were rare. The most common side effect was dyspepsia managed with proton pump inhibitors. Although we were initially concerned with kidney abnormalities, none were observed, and we stopped routine acquisition of renal scans prior to initiating RT. The oldest patient was treated at 91 years of age for stage IAE disease. He was last seen for follow-up 15 years later at 106 years of age and remained free of disease.
Two of 178 patients (1%) experienced a grade 3 complication of esophageal stricture requiring dilation. Both patients received standard 30-Gy radiation in 20 fractions. The first patient to experience toxicity was a patient who developed a local failure 1.1 years after completing radiotherapy. She subsequently developed nausea and vomiting and underwent dilation for esophageal stricture 2 years after completing radiation. The other patient underwent esophageal dilation 8 years after RT.
Two of 178 patients (1.6%) developed secondary malignancies within the radiation field. Both patients developed pancreatic cancer that was diagnosed 7.7 and 11.1 years following RT. One patient who initially presented with stage IV GML developed gastric adenocarcinoma 1.2 years after treatment and was considered to be a metachronous primary. He was subsequently treated with gastrectomy and remains alive 21 years after treatment.
An additional 17 of 178 patients (10%) developed additional solid malignancies that were outside of the radiation field and with shorter latency time (median, 4.2 years). These non-RT–related malignancies included cutaneous (5 patients), bladder (3 patients), breast (3 patients), colon (3 patients), and prostate (2 patients) cancer.
Discussion
This study is the largest and most comprehensive review of RT for gastric MALT performed at a single institution. The statistical results support the outcomes of similarly structured studies. RT is a highly safe and effective treatment for gastric MALT lymphoma, with excellent local disease control and rare treatment-related toxicities.
The greatest strength of this study is the homogenous and consistent therapeutic regimen carried out at a single institution with excellent long-term follow-up including EGDs. Pathologic confirmation was performed in-house for all patients in the study, and patients were followed at this institution for up to 23 years. All but 6 patients were monitored for relapse by EGD testing in the months and years following treatment.
Pathologic assessment for disease response is challenging. It can be difficult to differentiate MALT lymphoma from nonspecific features, such as inflammation, superficial erosion, or ulceration; and high rates of interobserver variation have been reported for posttreatment biopsy specimens.13 Our consistent evaluation with reliable hematopathology experts from Memorial Sloan Kettering Cancer Center is a significant strength of our study and enabled us to detail the natural history of EGD abnormalities. Three of 20 (15%) EGD failures resolved with observation alone, and only 39% of patients found to have atypical lymphoid cells not diagnostic of lymphoma (Wotherspoon score 3-4) subsequently progressed to local failure with true MALT lymphoma.
The majority of patients (95%) were found to be free of disease on first biopsy, and we continue to recommend EGD biopsy 3 months after completing therapy. Median time to local disease failure was 13.7 months and the 5- and 10-year cumulative incidence of local treatment failure was 7% and 11%, respectively. Given the low transformation rate and overall low lymphoma-related mortality, a less rigid EGD follow-up schedule can be safely considered. We recommend annual EGDs for the first 5 years and a decreasing frequency for asymptomatic patients with no suspicious pathologic abnormalities for up to 10 years posttreatment, considering the personal comfort levels of patients and their providers. Some patients with pathologic abnormalities experienced spontaneous resolution with observation alone. Therefore, it recommended to repeat evaluation of EGD abnormalities at 6 to 12 months before considering salvage therapies.
Limitations of this study include the retrospective nature of the chart review and the small number of lymphoma-related events. However, these limitations are offset by Memorial Sloan Kettering Cancer Center being a cancer-specific institute. Second malignancies and treatment-related toxicities are well captured by our medical record system, as reflected by the robust lymphoma physician follow-up times for PFS (median, 5.3 years; range, 1.4 months-23 years).
Our data are consistent with survival and response rates reported in other reports.9-12 The low incidence of treatment-related toxicities is also consistent with data reported in the literature. A prospective series of 53 patients who underwent long-term endoscopic follow-up for moderate-dose radiotherapy for GML found that, although rates of metachronous gastric carcinoma were noted to be higher in patients with gastric lymphoma compared with the general population, this was true regardless of treatment modality (antibiotics, RT, or immune/chemotherapy) and was attributed to associated H pylori gastritis.9 A recent analysis of 2996 patients from the Surveillance, Epidemiology, and End Results database found that radiation improved survival among patients with stage I gastric MALT without increasing the risk of cardiac death.12
OS was significantly associated with patient age at diagnosis and was not affected by local or distant disease failure. The comparable OS of patients who experienced local and distant disease failures attests to the excellent salvage therapies, including systemic treatment, surgery, and re-irradiation, which all remain available to patients following definitive radiation for GML. The decline in OS at 10 and 15 years reflects aging of the patient population (at 15 years, the median age of our patient cohort is 78.5 years), and it should be viewed in context with the expected life expectancy of the US population (78.6 years).17 We address this limitation of low lymphoma-related mortality by focusing our analysis on local and distant disease failure. Importantly, we considered death a competing risk for disease progression using a Fine and Gray competing risks regression.
This comprehensive study provides credible and compelling support for the continued use of RT as a safe and highly effective therapy for gastric MALT lymphoma. Acute or late treatment- related toxicities were very rare, demonstrating the safety of this treatment, even in elderly patients.
Since the original report of RT as definitive therapy for GML,8 significant advances in radiation technology have improved our ability to deliver treatment with less toxicity. In our original series of 17 patients,8 85% (15/17) were treated using anterior and posterior fields. Since then, RT has evolved to using 4-field 3-dimensional conformal radiation (3DCRT) and IMRT, with incremental improvements in the dose to the kidney and liver.18 Recent studies have demonstrated that CT IMRT can account for interfractional variation in stomach volume.19 Deep-inspiration breath-hold has also been shown to significantly decrease the radiation dose to the heart.20 A recent series of 32 patients demonstrated that a reduced dose of 24 Gy could be used effectively, with no disease failures identified at 2 years.21 These excellent outcomes compel the investigation of radiation dose deintensification with long-term follow-up. Ultralow-dose radiation with 4 Gy is under investigation in a clinical trial, and we are excited to see these continued advances in delivering radiation treatments with less toxicity.
Data sharing requests should be sent to Joachim Yahalom (yahalomj@mskcc.org).
Acknowledgments
This work was supported by the Connecticut Cancer Foundation and a Memorial Sloan Kettering Cancer Center Support Grant (P30 CA008748 from the National Insitutes of Health, National Cancer Institute).
Authorship
Contribution: J.Y. and A.J.X. designed the study and all authors performed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: A.D. has received personal fees from Roche, Corvus Pharmaceuticals, Physicians’ Education Resource, Seattle Genetics, PeerView Institute for Medical Education, Takeda, and EUSA Pharma and research grants from the National Cancer Institute and Roche. The remaining authors declare no competing financial interests.
Correspondence: Joachim Yahalom, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: yahalomj@mskcc.org.