Visual Abstract
TO THE EDITOR:
The relative efficacy of autologous hematopoietic cell transplant (auto-HCT) vs chimeric antigen receptor T-cell (CAR-T) therapy in patients with diffuse large B-cell lymphoma (DLBCL) who achieve a partial remission (PR) after salvage chemotherapy is not known. Using the Center for International Blood & Marrow Transplant Research registry database, we identified adult DLBCL patients who received either an auto-HCT (2013-2019) or CAR-T treatment with axicabtagene ciloleucel (2018-2019) while in a PR by computed tomography or positron emission tomography scan. We compared the clinical outcomes between the 2 cohorts using univariable and multivariable regression models after adjustment for relevant baseline and clinical factors. In the univariable analysis, the 2-year progression-free survival (52% vs 42%; P = .1) and the rate of 100-day nonrelapse mortality (4% vs 2%; P = .3) were not different between the 2 cohorts, but consolidation with auto-HCT was associated with a lower rate of relapse/progression (40% vs 53%; P = .05) and a superior overall survival (OS) (69% vs 47%; P = .004) at 2 years. In the multivariable regression analysis, treatment with auto-HCT was associated with a significantly lower risk of relapse/progression rate (hazard ratio = 1.49; P = .01) and a superior OS (hazard ratio =1.63; P = .008). In patients with DLBCL in a PR after salvage therapy, treatment with auto-HCT was associated with a lower incidence of relapse and a superior OS compared with CAR-T. These data support the role of auto-HCT as the standard of care in transplant-eligible patients with relapsed DLBCL in PR after salvage therapy.
B-cell maturation antigen targeted chimeric antigen receptor T-cell therapy (BCMA-CAR-T) for multiple myeloma (MM) treatment is associated with immunotoxicity,1-6 including cytokine release syndrome (CRS), neurotoxicity, and macrophage activation syndrome (MAS). MAS manifestations overlap considerably with hemophagocytic lymphohistiocytosis (HLH).7,8 Prior studies have described individual cases of HLH/MAS in CD19-CAR-T recipients.9,10 In a CD22-CAR-T study, HLH/MAS was reported in 32.7% of participants11 who exhibited distinct cytokine profiles and were less responsive to tocilizumab compared with patients with CRS alone.12 Few additional reports exist on HLH/MAS following CAR-T.
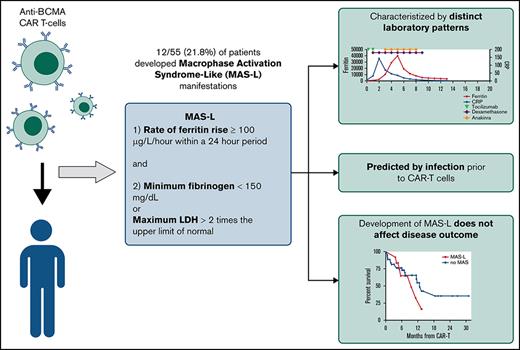
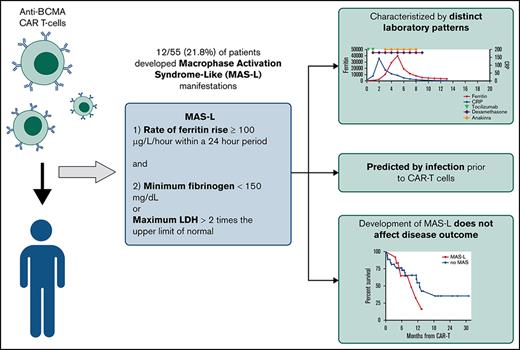
Applying traditional HLH criteria to post-CAR-T MAS is challenging because of reliance on cytopenias, which can be confounded by effects of malignancy or lymphodepletion, and fever, which can overlap with CRS.13,14 A proposed CAR-T-related HLH/MAS definition has relied on end-organ damage,15 which limits early detection of MAS. Therefore, we sought to develop novel criteria for MAS-like (MAS-L) disease following CAR-T.
We reviewed patients with BCMA-CAR-T MM at University of California San Francisco from 1 November 2017 through 1 May 2020 under an institutional review board-approved study. We defined MAS-L using the following criteria developed via physician consensus: (1) ferritin rise ≥100 μg/L/h within a 24-hour period and (2) minimum fibrinogen <150 mg/dL or maximum lactate dehydrogenase >2 times the upper limit of normal, during hospitalization (minimum 14 days) following CAR-T. We considered multiple macrophage activation markers, including triglycerides, soluble interleukin-2 receptor, and natural killer cell activity, but prioritized markers with rapid turnaround time to facilitate early MAS-L identification.
Infection was defined as culture positivity, febrile neutropenia, or clinical suspicion prompting antimicrobial initiation within 30 days before CAR-T. CRS and neurotoxicity were graded per consensus criteria.16 Hematologic count recovery was assessed at day 30, 60, and 90 after CAR-T as previously described.17 Disease response rate was determined per International Myeloma Working Group criteria.18
Continuous variables were compared using Wilcoxon rank-sum tests and binary outcomes were compared using Fisher exact tests. Unadjusted covariate effects were evaluated using logistic regressions. Overall and relapse-free survival (OS, RFS) were estimated using Kaplan-Meier curves and compared using log-rank tests. Analysis was performed using R 3.6.3 (Vienna, Austria.)
Fifty-five patients were treated with BCMA-CAR-T for MM; 12/55 (21.8%) met MAS-L criteria. Median follow-up time was 8.8 months. Compared with historical HLH/MAS definitions, our criteria captured more patients. Using the HLH-200413 and CAR-T-related HLH/MAS criteria,15 10 (18.2%) and 1 patient (2%) met criteria, respectively. All patients who met historical criteria also met our proposed criteria.
Patients with and without MAS-L had similar disease and treatment characteristics (Table 1). A higher proportion of patients with MAS-L had an infection before CAR-T (75% vs 9.3%, P < .001). Median time from infection to CAR-T was 13 days (range, 8-29); there was no difference between MAS-L vs non-MAS-L patients.
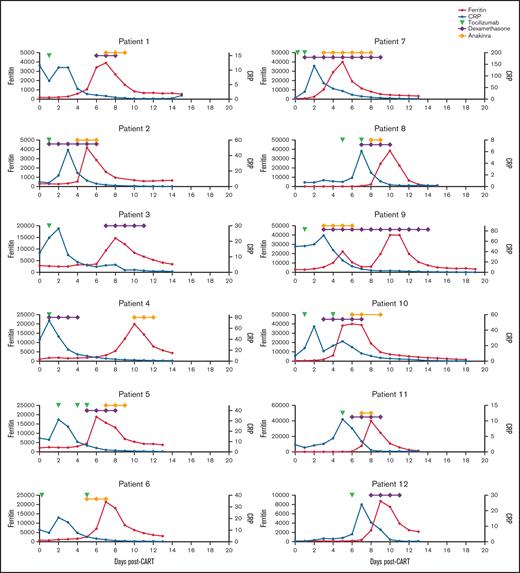
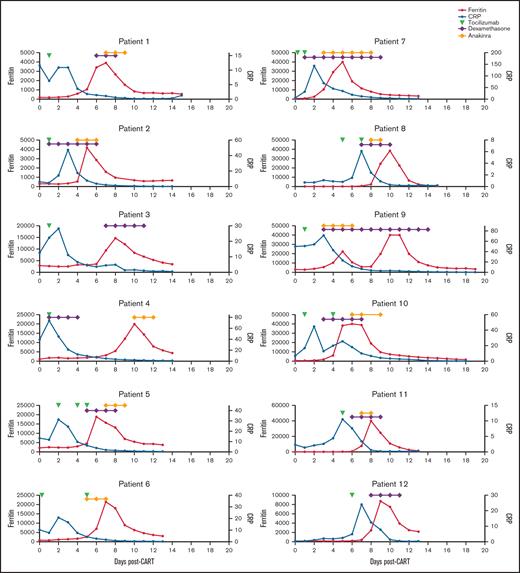
Patients who developed MAS-L followed similar disease trajectories. All 12 patients developed CRS, characterized by fever and elevated C-reactive protein (CRP), and received tocilizumab. Following tocilizumab, CRP decreased and patients developed rapid ferritin rise (Figure 1). Although many patients with MAS-L also had CRS with CRP elevation, the subsequent rapid ferritin rise was not observed (supplemental Figure 1).
Ferritin, CRP, and treatment over time in patients who developed macrophage activation-like syndrome (MAS-L) following CAR T-cell therapy. Ferritin (red), C-reactive protein (CRP) (blue), and administration of tocilizumab (green triangle), systemic steroids (purple triangle), and/or anakinra (yellow) over time for the 12 patients who developed MAS-L.
Ferritin, CRP, and treatment over time in patients who developed macrophage activation-like syndrome (MAS-L) following CAR T-cell therapy. Ferritin (red), C-reactive protein (CRP) (blue), and administration of tocilizumab (green triangle), systemic steroids (purple triangle), and/or anakinra (yellow) over time for the 12 patients who developed MAS-L.
Per our definition, patients with MAS-L had significantly higher maximum ferritin, higher lactate dehydrogenase, and lower fibrinogen. These patients also had significantly higher d-dimer, aspartate aminotransferase, and lower CRP (Table 1). In univariate logistic regression of patient factors and ferritin, CRP, and d-dimer before CAR T cells, only prior infection predicted MAS-L (hazard ratio, 29.2; 95% confidence interval, 5.54-154; P < .001) (supplemental Table 1).
During hospitalization, patients with MAS-L had lower platelet (14 vs 49 × 103/μL, P = .001) and white blood cells (0.25 vs 0.50 × 103/μL, P = .008) nadirs compared with patients with MAS-L; there was no difference in minimum hemoglobin or absolute neutrophil count. Similarly, at day 30 after CAR-T, fewer patients with MAS-L demonstrated hemoglobin (58.3% vs 92.1%, P = .01) and platelet (41.7% vs 78.9%, P = .03) recovery. By day 90 after CAR-T, there was no difference in count recovery for any cell line between patients who developed MAS-L vs those who did not (supplemental Figure 2).
All 12 patients with MAS-L received tocilizumab for CRS. Following tocilizumab, CRS resolved in 6 patients and persisted in 6 patients. Systemic steroids were given to treat CRS, neurotoxicity, and neurotoxicity plus MAS-L in 6, 3, and 2 patients, respectively. Of the 12 MAS-L patients, 10 received anakinra for MAS-L; following anakinra, ferritin decreased by >50% within 48 hours in 6 patients and within 72 hours in all 10 patients.
A similar proportion of patients with MAS-L vs without MAS-L developed any CRS (100% vs 84%, P = .33) and ≥grade 2 CRS (50% vs 50%, P = 1). Of the patients who developed CRS, those with MAS-L had longer CRS duration (5.4 vs 3.7 days, P = .03). Neurotoxicity was more common in patients with MAS-L (42% vs 14%, P = .05). A greater proportion of patients with MAS-L received tocilizumab (100% vs 70%, P = .05), steroids (92% vs 27%, P < .001), and anakinra (83% vs 2.3%, P < .001). Patients with MAS-L had longer hospitalizations (median, 21 vs 19 days; P = .009) and a greater proportion required intensive care unit-level care (27% vs 2%, P = .02).
Overall response rate was 100% vs 86% for patients with MAS-L vs those without MAS-L, respectively (P = .05). OS and RFS between the 2 groups were similar. One-year OS was 65.2% vs 90.6% (P = .16) and RFS was 35.4% vs 54.7% (P = .37) for patients with MAS-L vs those without MAS-L, respectively (supplemental Figure 3).
We developed simplified MAS-L criteria following BCMA-CAR-T and identified a 21.8% MAS-L incidence among 55 patients. Although patients with MAS-L required heightened monitoring, this immunotoxicity may be mitigated with steroids, anakinra, and supportive care. We also demonstrate that patients with MAS-L had similar improved disease outcomes compared with patients without MAS-L, a pattern also observed in CRS.19,20 Our findings suggest that once initial immunotoxicity is treated, patients with MAS-L have excellent outcomes. Although ideal treatment of MAS-L remains unknown, our practice is to initiate anakinra for patients meeting MAS-L criteria.
Interestingly, we found that patients with MAS-L had significantly lower CRP compared with patients without MAS-L. This differs from CRS, where CRP correlates positively with CRS following CD199,21,22 and BCMA-CAR-T.3-5 In addition, we found antecedent infections were strongly associated with MAS-L. Outside of CAR-T, infections are an established trigger for MAS/HLH23; the role in other post-CAR-T immunotoxicities is unknown.
The pathophysiology of MAS-L remains poorly understood. Broadly, immunotoxicity results from the inflammatory cytokine milieu produced by CAR-T and bystander macrophage activation.24,25 It is unclear whether MAS-L exists along the continuum of CRS or represents a distinct immunologic process. Our findings, including the inverse correlation of CRP and MAS-L development, suggest the pathophysiology of MAS-L may be distinct.
Our MAS-L criteria were based on physician consensus, a methodology with limitations as all possible laboratory values and thresholds were not analyzed given risk of overfitting in a small cohort. Our criteria require validation, which will be key in identifying additional predictors of MAS-L, including the role of CAR-T features, such as antigen binding site or costimulatory molecules. Furthermore, whether our definition applies to other CAR-T targets remains unknown. Future directions include prospective correlation with cytokines, which may identify biomarkers predictive of MAS-L development and provide insight into the underlying pathophysiology.
By defining MAS-L, future studies can better identify potential candidates for interventions. Animal models have demonstrated macrophage secretion of interleukin-1-β contributes to post-CAR-T immunotoxicity, and anakinra may mitigate this effect.24,26 Currently, there are ongoing trials of anakinra following CAR-T (NCT04205838, NCT04148430). Stratifying outcomes by patients meeting MAS-L criteria could provide additional insight into the benefit of this and other interventions.
Contribution: V.E.K.: data collection, data validation, data analysis, figure development, manuscript writing, manuscript revision and editing; C.W.: data collection, data validation, data analysis, manuscript revision and editing; C.Y.H.: data analysis, figure development, manuscript revision and editing; S.K.T.: data collection, data validation, data analysis, manuscript revision and editing; J.W.: conceptualization, data collection, manuscript revision and editing; T.G.M.: conceptualization, data collection, manuscript revision and editing; N.S.: conceptualization, data collection, manuscript revision and editing; and S.W.W.: conceptualization, data collection, data validation, data analysis, manuscript revision and editing.
Conflict-of-interest disclosure: J.W. discloses conflicts of interest with Amgen (consulting), Celgene (consulting), Janssen (consulting), Novartis (consulting), and Takeda (consulting). T.G.M. discloses conflicts of interest with Amgen (research), GSK (consulting), Janssen (research) Juno (consulting), Roche (consulting), Sanofi (research), and Seattle Genetics (research). N.S. discloses conflicts of interest with Amgen (consulting), Bluebird (research), BMS (consulting), Celgene (consulting), Genentech (research), GSK (consulting), Indapta (equity), Janssen (research), Karyopharm (consulting), Kite (consulting), Nektar (consulting), Nkarta (consulting), Oncopeptides (research), Poseida (research), Precision biosciences (consulting), Seattle Genetics (research), Surface Oncology (research), Sutro (research), and Teneobio (consulting). S.W.W. discloses conflicts of interest with Celgene (research), Fortis (research), Genentech (research), Janssen (research), Juno (research), Sanofi (consulting), Amgen (consulting), and GlaxoSmithKline (research). The remaining authors declare no competing financial interests.
Correspondence: Vanessa E. Kennedy, Division of Hematology and Oncology, Department of Medicine, University of California San Francisco, 505 Parnassus Ave, Rm 1286, Mailbox 1270, San Francisco, CA 94143-1270; email: vanessa.kennedy@ucsf.edu.
References
Author notes
Requests for data sharing may be submitted to Vanessa E. Kennedy (vanessa.kennedy@ucsf.edu) or Sandy W. Wong (sandyw.wong@ucsf.edu).
The full-text version of this article contains a data supplement.