Key Points
We report a case of VITT following Ad26.COV2.S COVID-19 vaccination without radiographically demonstrable thrombosis at presentation.
Early VITT recognition and treatment with nonheparin anticoagulation can prevent severe thrombotic complications.
Abstract
We report a case of vaccine-induced immune thrombotic thrombocytopenia (VITT) in a young man diagnosed 13 days after Ad26.COV2.S COVID-19 (Johnson & Johnson/Janssen) vaccination. He presented to us with 5 days of progressive left leg pain, thrombocytopenia, hypofibrinogenemia, and markedly elevated d-dimers, but without radiographically demonstrable thrombosis. Despite negative imaging, we initiated treatment of presumptive VITT given the striking clinical picture that included the timing of his recent adenovirus-based COVID-19 vaccine, leg symptoms, marked thrombocytopenia, and consumptive coagulopathy. He received intravenous immune globulin, prednisone, and argatroban and was discharged 7 days later much improved. His positive platelet factor 4 enzyme-linked immunosorbent assay antibody test returned after treatment was initiated. To our knowledge, this is the first reported case of VITT following Ad26.COV2.S vaccination presenting without radiographically demonstrable thrombosis. Our patient highlights the importance of knowing vaccine status and initiating treatment as soon as possible in the right clinical setting, even in the absence of radiographic evidence of thrombus. Early VITT recognition and treatment provide an opportunity to prevent serious thrombotic complications.
Introduction
A rare syndrome involving thrombosis and thrombocytopenia has been reported following COVID-19 vaccination with a recombinant adenoviral vector encoding the SARS-CoV-2 spike protein, including 41 reported cases following ChAdOx1 nCoV-19 (AstraZeneca) vaccination1-5 and 28 cases following administration of >8.7 million Ad26.COV2.S (Johnson & Johnson/Janssen) vaccinations in the United States.6-8
Based on these reports, a new pathologic entity has been proposed, variably termed thrombosis with thrombocytopenia syndrome and vaccine-induced immune thrombotic thrombocytopenia (VITT).9 The cases are notable for significant thrombocytopenia and thrombosis, often in atypical locations including cerebral venous sinus and intraabdominal thrombosis in the portal, splenic, hepatic, and splanchnic veins. Some patients experienced arterial thrombosis, and many have had multiple thromboses.1-3,5,6 As of 7 May 2021, 3 (11%) of the 28 reported and published cases of VITT following Ad26.COV2.S vaccination6,7,10,11 and 16 (39%) of the 41 reported patients with VITT following ChAdOx1 nCoV-19 vaccination1-5 had died of this syndrome; many others suffered significant morbidity. Because of the clinical and laboratory resemblance of VITT to autoimmune or spontaneous heparin-induced thrombocytopenia (HIT),12 initial providers looked for and found anti-platelet factor 4 (PF4) antibodies in their patients despite lack of prior heparin exposure.1-3
These case reports have led to guidelines to help identify patients with VITT. Current working case definitions include: (1) SARS-CoV-2 vaccination with recombinant adenoviral vector in the prior 5 to 30 days; (2) venous or arterial thrombosis; (3) thrombocytopenia; and (4) presence of anti-PF4 antibodies.8,9 In addition, exceedingly high d-dimer levels have been universally reported in VITT patients following ChAdOx1 nCoV-19 vaccination,1-3 and low fibrinogen levels are common, suggesting a coincident consumptive coagulopathy similar to severe autoimmune HIT.12,13 We present a case of VITT in a man who presented 13 days after receiving the Ad26.COV2.S vaccine without radiographically demonstrable thrombosis who was successfully treated with intravenous immune globulin (IVIG), glucocorticoids, and non-heparin anticoagulation.
Case description
Eight days after receiving the Ad26.COV2.S vaccine, an otherwise healthy 32-year-old man developed left leg pain, erythema, and gravity-dependent venous distention. Three days later, he presented to an urgent care center. A lower extremity ultrasound was negative for deep venous thrombosis and a complete blood count showed thrombocytopenia with a platelet count of 61 × 109/L. Because of the unexplained thrombocytopenia, he returned the following day. A repeat complete blood count showed a platelet count of 52 × 109/L that remained unexplained. The next day, he presented to our emergency department with worsening leg pain. He was otherwise asymptomatic postvaccination.
On presentation, he reported left leg pain regardless of position, and physical examination revealed left leg venous distention and erythema upon standing. When asked about COVID vaccine status, he reported that he had not previously been asked. Laboratory evaluation demonstrated worsening thrombocytopenia with a platelet count of 43 × 109/L, elevated prothrombin time of 16.3 seconds (international normalized ratio 1.4), elevated activated partial thromboplastin time of 35 seconds, decreased fibrinogen of 110 mg/dL, and markedly elevated d-dimer of >14 000 ng/mL. A lower extremity ultrasound was again negative for thrombosis from the proximal femoral vein to the ankle. Chest/abdomen/pelvis computed tomography with contrast demonstrated no thrombosis. Brain magnetic resonance imaging revealed patent sinuses without thrombosis. VITT was suspected given his vaccination history, symptoms, and laboratory findings. He was admitted and started on 1 g/kg of IVIG daily for 2 days to increase his platelet count and given 1 mg/kg of prednisone.
The following day, his pain worsened. Despite lack of radiographically demonstrable thrombosis, anticoagulation with argatroban was started. Later that day, the enzyme-linked immunosorbent assay (ELISA) for anti-PF4 antibodies (Immucor, Norcross, GA) drawn the day prior returned strongly positive at 2.79 optical density (OD) units (normal, ≤0.40) in the absence of added heparin; the assay was negative in the presence of high-dose heparin (100 U/mL). The confirmatory washed-platelet, heparin-induced platelet activation assay was positive.
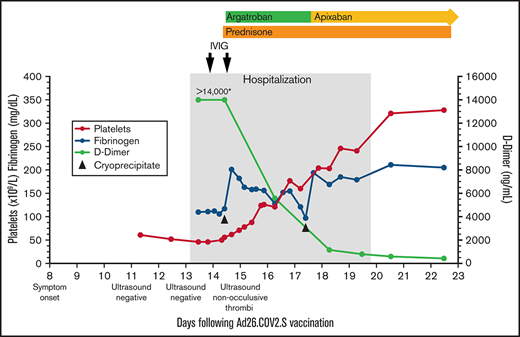
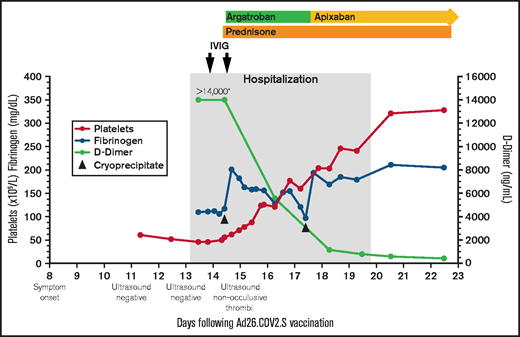
His platelets rose quickly in response to the IVIG (Figure 1), allowing us to start anticoagulation. Within 24 hours of starting argatroban, his pain began to improve. Given persistent venous distention, a repeat lower extremity ultrasound was performed that demonstrated reflux throughout the deep veins and the greater saphenous vein from the saphenofemoral junction to the mid-calf. The exam also identified nonocclusive thrombi in the left distal popliteal vein and peroneal vein, which were not present on admission.
Platelet count, fibrinogen, D-dimer, and treatment over time. The X-axis indicates the days following Ad26.COV2.S vaccination. The left Y-axis indicates platelet count and fibrinogen concentration; the right Y-axis indicates D-dimer concentration. Normal range for platelet count (140-450 ×109/L), fibrinogen (202-430 mg/dL), d-dimer (<500 ng/mL). *>14 000 is the maximum d-dimer value reported by our laboratory. Hospitalization, the gray-shaded column, indicates the time from presentation to our emergency department until discharge from the hospital. IVIG (1 g/kg) was administered to increase platelet count to allow for safe initiation of anticoagulation. Cryoprecipitate (first dose 10 units; second dose 5 units) was given to improve fibrinogen levels to allow for safe initiation of anticoagulation. Prednisone 1 mg/kg per day with taper. Therapeutic argatroban was dosed for target activated partial thromboplastin time of 60 to 80 seconds. Apixaban 10 mg twice daily ×7 days followed by 5 mg twice daily.
Platelet count, fibrinogen, D-dimer, and treatment over time. The X-axis indicates the days following Ad26.COV2.S vaccination. The left Y-axis indicates platelet count and fibrinogen concentration; the right Y-axis indicates D-dimer concentration. Normal range for platelet count (140-450 ×109/L), fibrinogen (202-430 mg/dL), d-dimer (<500 ng/mL). *>14 000 is the maximum d-dimer value reported by our laboratory. Hospitalization, the gray-shaded column, indicates the time from presentation to our emergency department until discharge from the hospital. IVIG (1 g/kg) was administered to increase platelet count to allow for safe initiation of anticoagulation. Cryoprecipitate (first dose 10 units; second dose 5 units) was given to improve fibrinogen levels to allow for safe initiation of anticoagulation. Prednisone 1 mg/kg per day with taper. Therapeutic argatroban was dosed for target activated partial thromboplastin time of 60 to 80 seconds. Apixaban 10 mg twice daily ×7 days followed by 5 mg twice daily.
Over the next few days, his platelets rose steadily, d-dimers fell precipitously, and he was transitioned from argatroban to apixaban (Figure 1). On the sixth hospital day, repeat anti-PF4 ELISA remained positive (OD 2.68) in the absence of added heparin; negative in the presence of high-dose heparin (100 U/mL). The confirmatory washed-platelet, heparin-induced platelet activation assay was now negative. On the seventh hospital day, he was discharged home. Seven days after discharge, the anti-PF4 ELISA remained positive (OD 2.32), the washed-platelet, heparin-induced platelet activation assay remained negative, and his international normalized ratio, activated partial thromboplastin time, fibrinogen, platelet count, and d-dimer had normalized.
Methods
The methodology combined retrospective chart review as well as prospective data collection following hospital presentation. The patient consented for this case report.
Results and discussion
The understanding of the clinical spectrum and natural history of VITT is evolving.8,9,14 Given our patient’s presenting history and examination, thrombocytopenia, markedly elevated d-dimers, and low fibrinogen, we initiated treatment of presumed VITT despite the absence of radiographically demonstrable thrombosis and before his anti-PF4 ELISA resulted. To our knowledge, this is the first reported case of VITT following Ad26.COV2.S vaccination without radiographically demonstrable thrombosis at presentation.
Our patient’s clinical course suggests that early VITT recognition and immediate treatment initiation provide a window of opportunity wherein non-heparin-based anticoagulation may prevent serious thrombotic complications. It also highlights the critical importance of knowing COVID-19 vaccine history, something that he was not asked when he first presented with leg symptoms and “unexplained” thrombocytopenia. A recent report describes a woman who presented with petechiae 9 days after ChAdOx1 nCoV-19 vaccination and was found to have thrombocytopenia, hypofibrinogenemia, markedly elevated d-dimer, but no demonstrable thrombosis at presentation. She was treated with IVIG and non-heparin anticoagulation and was discharged without known thrombosis development, but repeat imaging was not reported.4 That case and ours demonstrate that there is a window wherein early treatment can prevent serious VITT complications. We suggest that patients who present with thrombocytopenia and markedly elevated d-dimers 5 to 30 days following adenovirus-based COVID-19 vaccination receive non-heparin anticoagulation independent of radiographically demonstrable thrombosis and pending anti-PF-4 ELISA results.
On the day of presentation, our patient’s anti-PF4 ELISA and the confirmatory platelet activation assay were positive. Five, 13, and 43 days later, the anti-PF4 ELISA remined strongly positive, but the confirmatory platelet activation assay was negative, the latter likely from the impact of IVIG on the platelet activation assay.15,16 It will be important to define the time course for positive anti-PF4 antibodies in patients with VITT and to understand risk of long-term thrombosis, spontaneous recurrence, and safety of future heparin or adenovirus-based vaccine exposure.
VITT remains a rare complication. However, given the large number of people anticipated to receive adenovirus-based COVID-19 vaccination, it is critical that clinicians recognize the presenting clinical spectrum of this emerging syndrome. We need to anticipate that VITT will have various presentations, much like HIT itself.17 The diagnostic criteria for VITT will need continued refinement, including criteria that support early intervention. Timely recognition will be essential to prevent catastrophic complications and will require knowing the patient’s vaccine history.
Acknowledgment
The authors thank Agnes Y. Y. Lee, University of British Columbia, for critical reading of the manuscript.
Authorship
Contribution: V.E.K., C.C.W., P.C., and A.D.L. provided concept and design; V.E.K., C.C.W., J.M.H., T.P., and S.B. acquired data, including patient management; V.E.K., C.C.W., S.B., P.C., A.D.L. wrote and revised manuscript; LR provided expert interpretation of the vascular ultrasound.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Vanessa E. Kennedy, Department of Medicine, University of California San Francisco, 505 Parnassus Ave, Room M1286, Mailbox 1270, San Francisco, CA 94134; e-mail: vanessa.kennedy@ucsf.edu.
References
Author notes
The original data are available by e-mail request to Vanessa Kennedy (vanessa.kennedy@ucsf.edu).