Key Points
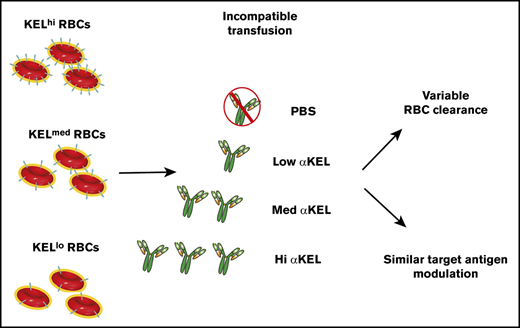
KEL antigen density can dictate the rate and overall magnitude of RBC clearance following an incompatible transfusion.
Antibody-induced loss of target antigen from the surface of transfused RBCs occurs independent of KEL RBC antigen density.
Abstract
Incompatible red blood cell (RBC) transfusion can result in life-threatening transfusion complications that can be challenging to manage in patients with transfusion-dependent anemia. However, not all incompatible RBC transfusions result in significant RBC removal. One factor that may regulate the outcome of incompatible RBC transfusion is the density of the incompatible antigen. Despite the potential influence of target antigen levels during incompatible RBC transfusion, a model system capable of defining the role of antigen density in this process has not been developed. In this study, we describe a novel model system of incompatible transfusion using donor mice that express different levels of the KEL antigen and recipients with varying anti-KEL antibody concentrations. Transfusion of KEL+ RBCs that express high or moderate KEL antigen levels results in rapid antibody-mediated RBC clearance. In contrast, relatively little RBC clearance was observed following the transfusion of KEL RBCs that express low KEL antigen levels. Intriguingly, unlike RBC clearance, loss of the KEL antigen from the transfused RBCs occurred at a similar rate regardless of the KEL antigen density following an incompatible transfusion. In addition to antigen density, anti-KEL antibody levels also regulated RBC removal and KEL antigen loss, suggesting that antigen density and antibody levels dictate incompatible RBC transfusion outcomes. These results demonstrate that antibody-induced antigen loss and RBC clearance can occur at distinct antigen density thresholds, providing important insight into factors that may dictate the outcome of an incompatible RBC transfusion.
Introduction
Red blood cell (RBC) transfusion represents a common therapeutic intervention in patients with chronic anemia.1-3 Although RBC transfusion is often beneficial, incompatible transfusions can result in the rapid removal of transfused RBCs with potential adverse consequences.4,5 Rapid, antibody-mediated removal of RBCs following an incompatible transfusion is rare. However, recent results suggest that this outcome may be more common in certain patient populations.4,6-12 Patients with transfusion-dependent conditions can present with life-threatening anemia and a complex-alloantibody profile that precludes prompt identification of compatible RBCs.13-19 In these situations, patients occasionally receive RBCs that are not fully compatible.20-22 Prior alloimmunization events can also prime patients to develop a recrudescent alloantibody response, which can result in the accelerated clearance of the transfused RBCs days to weeks following transfusion.23-31 Recent reports suggest that patients with sickle cell disease can be particularly prone to develop amnestic alloantibody responses.12,32 Often, these responses are accompanied by accelerated clearance of the transfused RBCs, potentially resulting in serious complications.12,32
Despite the potential consequences of incompatible RBC transfusions,33-37 the factors that determine the overall consequences of an incompatible RBC transfusion remain incompletely understood.38 RBC removal is not necessarily the absolute outcome of an incompatible transfusion.39 Although sophisticated in vitro approaches (such as the monocyte monolayer test40,41 ) exist that may aid in identifying incompatible transfusion scenarios where RBC clearance is likely to occur, the unexpected presentation of patients with life-threatening anemia can make it difficult to effectively use this platform in acute cases.42 When only incompatible RBCs are available and life-threatening anemia necessitates transfusion, optimal strategies that may prevent the removal of incompatible RBCs are needed but remain incompletely defined.43 Factors that regulate RBC removal following incompatible transfusion remain inadequately understood largely because of challenges studying incompatible RBC transfusions clinically and lack of suitable animal models to study incompatible transfusion biology in vivo.
Given the unpredictability in outcomes following incompatible RBC transfusions, deliberately transfusing incompatible RBCs in human subjects to define factors that may regulate RBC removal is not ethical. To overcome this challenge, recently developed preclinical models have provided an opportunity to begin examining the consequences of antibody engagement and the factors that may govern the outcomes of incompatible transfusion.33-37 Results obtained using these models suggest that the consequences of antibody engagement may be more complicated than simple antibody-mediated removal of target RBCs. Instead, competing processes appear to regulate the sensitivity of RBCs to antibody-mediated removal following an incompatible transfusion. These processes include common antibody effector functions (which may result in RBC clearance) and competing antibody-mediated removal of the target antigen from the RBC surface (antigen modulation).37,44-46 Antibody-mediated antigen modulation appears to render cells less sensitive to antibody-mediated removal and, therefore, may protect incompatible RBCs from antibody-mediated destruction.37 Complementary studies in patients who have received antibody-based drugs that target RBCs appear to corroborate these results, suggesting that antibody engagement of antigens on the RBC surface may alter the target antigen.47,48 However, a more complete understanding of the factors that regulate antigen modulation and subsequent RBC clearance is still needed.
Among factors that may regulate the consequences of incompatible RBC transfusion, the surface density of the target antigen has been suggested as a variable that may influence overall incompatible transfusion outcomes.49 However, although antibody engagement of different antigens may be more likely to result in RBC clearance, a variety of other variables (including other characteristics of the antigens themselves) may likewise contribute to these correlations. As a result, it is difficult to determine the potential impact of antigen density itself on the outcome of an incompatible transfusion. In this report, we use a novel murine system that couples RBCs that express varying levels of the KEL antigen to a model of incompatible transfusion. This approach is used to test the hypothesis that antigen density and antibody levels dictate the likelihood that RBC clearance will occur following an incompatible transfusion. Using this model, our results demonstrate that a combination of antigen density and anti-KEL antibody levels indeed dictate transfusion outcomes, from the rate and magnitude of RBC removal to selective loss of the target antigen.
Materials and methods
Mice
Wild-type C57BL/6 mice (WT) and transgenic mice expressing low (KELlo), intermediate (KELmed), and high (KELhi) human KEL glycoprotein36,50-53 were housed at Emory University Department of Animal Resources; all protocols and procedures were approved by the Emory University Institutional Animal Care and Use Committee.
Generation of anti-KEL antibodies and passive immunization
Anti-KEL antibodies were generated by transfusing KELmed RBCs into WT mice 3 times followed by evaluation of anti-KEL antibodies levels by flow cross-match as outlined previously.46,54,55 For passive immunization experiments, WT recipients were passively immunized with serum (2 µL [low], 6.25 µL [medium], or 24 µL [high]) containing anti-KEL antibodies before RBC transfusion as done previously.46,54,55
RBC isolation, labeling, and transfusion
Donor mice were bled as outlined previously.56-59 Each KEL RBC population was then labeled with lipophilic dye chloromethylbenzamido 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI), whereas WT RBCs were labeled with 3,3′-dihexadecyloxacarbocyanine perchlorate (DiO) as outlined previously.34,35 Recipient WT mice were transfused via lateral tail-vein injection as done previously.36,46,56-59
Blood collection, staining, and measurement of RBC clearance and serum cytokines
Recipient mice were bled at the indicated times, and RBCs were stained with anti-mouse immunoglobulin G (IgG; Jackson Immunoresearch) alone to detect bound antibody or anti-KEL antisera and anti-mouse IgG to detect total KEL antigen as outlined previously.46,55-59 Biotinylated monoclonal antibodies that either recognize an epitope present on C3/C3b/iC3b (designated C3/C3b/iC3b) or an epitope present on all forms of C3 (designated C3) were used, followed by detection using streptavidin antigen-presenting cell.46,57 Anti-KEL antibody binding and overall anti-KEL antibody levels were examination by flow cross-match as previously outlined.57-60 Percent RBC survival was measured by comparing the ratio of KEL RBCs (DiI) to WT RBCs (DiO) as done previously.34,35 Cytokines were measured using a flow cytometry-based kit (Biolegend) as outlined previously33 (see supplemental Methods for additional details).
Results
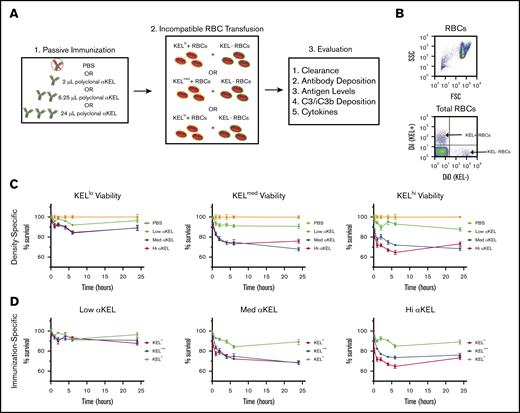
To define the impact of donor RBC antigen density on the consequence of an incompatible RBC transfusion, we first characterized distinct KEL transgenic founders for KEL antigen levels. KEL levels were unique on each RBC population isolated from different founders, with some expressing higher (KELhi), medium (KELmed), or lower (KELlo) levels of KEL, despite exhibiting similar size and surface expression of Ter119 (Figure 1A-B).51 To ensure that the observed differences did not reflect incomplete KEL saturation, we next examined the maximum anti-KEL antibody binding on each population. Similar to our initial results, the levels of KEL saturation differed with each population (Figure 1C), suggesting that KELhi, KELmed, or KELlo RBCs possess distinct KEL antigen levels. Additional examination of KEL antigen levels demonstrated that ∼5200 (KELhi), 3200 (KELmed), and 330 (KELlo) KEL antigens can be detected on each KEL+ RBC population (Table 1). Taken together, these results suggest that KEL antigen levels on each RBC population are unique. This could provide a tool to examine antigen density as an isolated variable when defining factors that may influence incompatible RBC transfusion outcomes.
Distinct KEL founders produce RBCs that express distinct levels of the KEL antigen. (A) Representative flow plot and histogram for distinct KEL RBC donor populations, including forward (FSC) and side scatter (SSC) profile gating strategy, staining for the KEL antigen with anti-KEL antibodies, and the level of Ter119 observed in each population. (B) Quantification of FSC profile, KEL antigen levels, and Ter119 staining in KELhi, KELmed, and KELlo RBCs. *KELlo vs B6, P < .001; **KELmed vs B6, P < .0001; and KELmed vs KELlo, P < .001; ***KELhi vs B6, P < .0001; KELhi vs KELlo, P < .0001; and KELhi vs KELmed, P < .0001. (C) Titration of anti-KEL polyclonal antibody against KELhi, KELmed, and KELlo RBCs followed by flow cytometric examination. (D) Examination of antibody binding to KELhi, KELmed, and KELlo RBCs following incubation with serum isolated from recipients that were passively immunized with the indicated doses of anti-KEL antibody. Error bars represent mean ± SEM. n = 3-5 mice per group. Each panel represents data reproduced 3 times.
Distinct KEL founders produce RBCs that express distinct levels of the KEL antigen. (A) Representative flow plot and histogram for distinct KEL RBC donor populations, including forward (FSC) and side scatter (SSC) profile gating strategy, staining for the KEL antigen with anti-KEL antibodies, and the level of Ter119 observed in each population. (B) Quantification of FSC profile, KEL antigen levels, and Ter119 staining in KELhi, KELmed, and KELlo RBCs. *KELlo vs B6, P < .001; **KELmed vs B6, P < .0001; and KELmed vs KELlo, P < .001; ***KELhi vs B6, P < .0001; KELhi vs KELlo, P < .0001; and KELhi vs KELmed, P < .0001. (C) Titration of anti-KEL polyclonal antibody against KELhi, KELmed, and KELlo RBCs followed by flow cytometric examination. (D) Examination of antibody binding to KELhi, KELmed, and KELlo RBCs following incubation with serum isolated from recipients that were passively immunized with the indicated doses of anti-KEL antibody. Error bars represent mean ± SEM. n = 3-5 mice per group. Each panel represents data reproduced 3 times.
In addition to target antigen density, alloantibody levels against a target alloantigen can differ between individuals,61 providing another variable that could potentially influence incompatible transfusion outcomes. To ensure that the antibody levels used in our model successfully saturated KEL at each antigen level, we passively immunized KEL− B6 recipients with varying levels of anti-KEL antibody, followed by examination of antibody binding to each KEL RBC population. Using this approach, a concentration of anti-KEL antibody capable of binding each KEL RBC population at saturating levels was identified (Figure 1D). This antibody level will be referred to as high anti-KEL antibody levels. Conditions were also developed in which medium and low levels of anti-KEL binding toward KELhi RBCs or KELmed RBCs were observed. These conditions included anti-KEL antibody levels that resulted in similar antibody binding to KELhi RBCs as was observed following KELmed RBC saturation (hereafter referred to as medium anti-KEL antibody levels) and anti-KEL antibody levels in which antibody binding to KELhi and KELmed RBCs were similar to antibody binding observed toward KELlo RBCs at saturation (low anti-KEL antibody levels).
Having RBC populations with distinct densities of KEL and accompanying recipient conditions that achieve saturating or nonsaturating KEL+ RBC binding, we next defined the impact of distinct antigen densities and anti-KEL antibody levels on incompatible RBC transfusion outcomes (Figure 2A). To accomplish this, each KEL RBC population was labeled with the lipophilic dye, DiI,34,35 to facilitate posttransfusion detection. As a control for antibody-independent RBC clearance, KEL− B6 RBCs were labeled with a fluorescently distinct lipophilic dye, DiO. Following fluorescent labeling, DiI RBCs positive for KEL and DiO KEL− RBCs were mixed at a defined ratio before transfusion. Each DiI+ KEL+ RBC and DiO+ WT (KEL−) RBC mixture was then transfused into either a nonimmunized or immunized recipient (Figure 2B). After transfusion, recipients were evaluated over time for subsequent KEL RBC removal.
KEL-specific RBC clearance varies by antigen density and passive immunization. (A) Schematic for experimental design. (B) Representative flow plot illustrating gating strategy of DiI-labeled KEL+ RBCs and DiO-labeled KEL-negative (KEL−) RBCs. (C) KEL+ RBC survival at 10 minutes, and 1, 2, 4, 6, and 24 hours posttransfusion in WT B6 mice immunized with a phosphate-buffered saline (PBS) control (orange) or with low (lo, green), medium (med, blue), or high (hi, red) dosages of anti-KEL antibodies (αKEL). (D) KELlo (green), KELmed (blue), or KELhi (red) RBC survival in WT B6 mice at 10 minutes, and 1, 2, 4, 6, and 24 hours after transfusion. The same RBC survival data are plotted in 2 ways to demonstrate the key variables of antigen density (C, density-specific) and concentration of passive immunization (D, immunization-specific). Error bars represent mean ± standard error of the mean (SEM). n = 5-7 mice per group. (C-D) represents data reproduced 3 times.
KEL-specific RBC clearance varies by antigen density and passive immunization. (A) Schematic for experimental design. (B) Representative flow plot illustrating gating strategy of DiI-labeled KEL+ RBCs and DiO-labeled KEL-negative (KEL−) RBCs. (C) KEL+ RBC survival at 10 minutes, and 1, 2, 4, 6, and 24 hours posttransfusion in WT B6 mice immunized with a phosphate-buffered saline (PBS) control (orange) or with low (lo, green), medium (med, blue), or high (hi, red) dosages of anti-KEL antibodies (αKEL). (D) KELlo (green), KELmed (blue), or KELhi (red) RBC survival in WT B6 mice at 10 minutes, and 1, 2, 4, 6, and 24 hours after transfusion. The same RBC survival data are plotted in 2 ways to demonstrate the key variables of antigen density (C, density-specific) and concentration of passive immunization (D, immunization-specific). Error bars represent mean ± standard error of the mean (SEM). n = 5-7 mice per group. (C-D) represents data reproduced 3 times.
Each KEL RBC population displayed similar RBC survival as coadministered B6 RBCs following transfusion into nonimmunized recipients. In contrast, KELhi, KELmed, or KELlo RBCs exhibited distinct clearance patterns following transfusion into anti-KEL immunized recipients. KELhi and KELmed RBCs displayed the most clearance among the 3 KEL+ RBC populations, with the highest level of clearance observed in WT recipients possessing either high or medium anti-KEL antibody levels (Figure 2C-D). KELlo RBC clearance could also be detected following transfusion into recipients with high or medium anti-KEL antibodies. However, KELlo RBC removal in these recipients occurred a reduced rate and overall magnitude when compared with KELhi and KELmed RBCs (Figure 2C-D). Transfusion of KELhi, KELmed, or KELlo RBCs into recipients that possessed the lowest levels of anti-KEL antibodies resulted in very little RBC clearance. These results suggest that RBC antigen density and recipient anti-KEL antibody levels may influence the magnitude of RBC clearance.
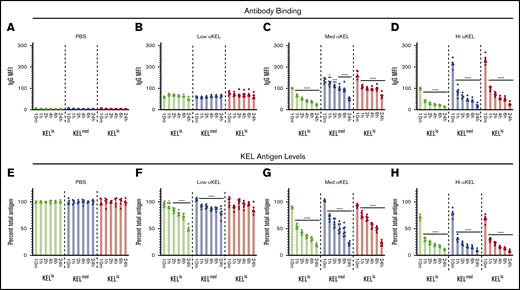
Although the differences observed in RBC clearance based on KEL antigen levels suggests the existence of a threshold that regulates incompatible RBC transfusion outcomes, whether anti-KEL antibodies efficiently engaged KEL on each RBC population in vivo remained unknown. To examine this, each KEL RBC population was stained for IgG deposition at the time points indicated posttransfusion. Using this approach, IgG could be detected on each KEL+ RBC population, where the level of IgG detected generally correlated with the initial KEL antigen levels and amount of anti-KEL antibody in each recipient (Figure 3A-D). KELhi and KELmed RBCs exhibited similar initial antibody levels, which were consistently higher than those detected on KELlo RBCs (Figure 3C-D). In contrast, each KEL+ population displayed similar levels of bound antibody following transfusion into immunized recipients with the lowest anti-KEL antibody levels (Figure 3B). No IgG could be detected on KEL RBCs transfused into nonimmunized recipients (Figure 3A), suggesting that anti-KEL antibodies specifically engaged KEL+ RBC populations following transfusion into immunized recipients. Despite distinct levels of antibody binding to each population, the level of bound antibody on each KEL RBC population did not reach similar levels observed in vitro (Figure 1D). The initial levels of antibody detected on the surface of each KEL+ RBC population not only failed to achieve the saturating levels observed in vitro, but these levels continued to decline over time (Figure 3C-D).
Transfusion of KELlo, KELmed, and KELhiRBCs into anti-KEL antibody immunized recipients results in antigen modulation. (A-D) RBCs with KELlo (green), KELmed (blue), and KELhi (red) were monitored posttransfusion for IgG deposition over time by flow cytometry at 10 minutes, and 1, 2, 4, 6, and 24 hours posttransfusion into PBS-treated control recipients (A) or recipients that possessed low (B), medium (med) (C), or high (hi) (D) αKEL levels. (E-H) RBCs with KELlo (green), KELmed (blue), and KELhi (red) were examined for total KEL antigen detected by flow cytometry at 10 minutes, and 1, 2, 4, 6, and 24 hours posttransfusion into PBS-treated (E) control recipients or recipients with low (F), medium (med) (G), or high (hi) (H) αKEL levels. Error bars represent mean ± SEM. n = 5-7 mice per group. Each panel represents data reproduced 3 times. *Significance indicated vs value at 10 minutes posttransfusion. P < .05.
Transfusion of KELlo, KELmed, and KELhiRBCs into anti-KEL antibody immunized recipients results in antigen modulation. (A-D) RBCs with KELlo (green), KELmed (blue), and KELhi (red) were monitored posttransfusion for IgG deposition over time by flow cytometry at 10 minutes, and 1, 2, 4, 6, and 24 hours posttransfusion into PBS-treated control recipients (A) or recipients that possessed low (B), medium (med) (C), or high (hi) (D) αKEL levels. (E-H) RBCs with KELlo (green), KELmed (blue), and KELhi (red) were examined for total KEL antigen detected by flow cytometry at 10 minutes, and 1, 2, 4, 6, and 24 hours posttransfusion into PBS-treated (E) control recipients or recipients with low (F), medium (med) (G), or high (hi) (H) αKEL levels. Error bars represent mean ± SEM. n = 5-7 mice per group. Each panel represents data reproduced 3 times. *Significance indicated vs value at 10 minutes posttransfusion. P < .05.
Previous studies suggested that antibody engagement of KEL may result in alterations to the KEL antigen.46,55 This raises the possibility that KEL antigen levels may change over time and therefore possibly account for the differences observed between in vitro saturation and initial antibody binding observed following incompatible RBC transfusion (Figure 3B-D). To test this, we next examined KEL antigen levels following each incompatible transfusion. KEL was detected on each KEL+ RBC population transfused into nonimmunized recipients and remained stable over the course of the experiment (Figure 3E). In contrast, the levels of KEL antigen declined rapidly following incompatible transfusion (Figure 3F-H). In this case, within 1 hour, levels of KEL dropped to <50% of the initial KEL antigen levels following transfusion into recipients with the highest anti-KEL antibody levels. This occurred regardless of the KEL+ RBC population (Figure 3H). Additionally, the rate of KEL antigen modulation in this group appeared to be similar regardless of the initial antigen level, with the percent loss showing comparable rates of removal over time (Figure 3H). In contrast, rates of antigen loss for KELhi and KELmed RBCs appeared to be slightly reduced when compared with KELlo RBCs following transfusion into recipients with medium anti-KEL antibody levels (Figure 3G). Finally, despite similar antibody binding observed initially, very little KEL antigen loss was observed on KELhi or KELmed RBCs following transfusion into recipients that received the lowest level of anti-KEL antibodies, whereas KELlo RBCs continued to experience a gradual decline in antigen levels when exposed to these same low anti-KEL antibody levels (Figure 3F).
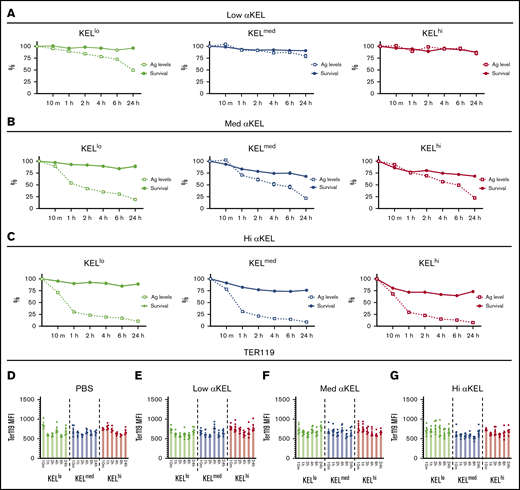
Reductions in antigen levels appeared to correlate with reduced RBC clearance over time, suggesting that loss of antigen may provide protection against RBC clearance or may simply reflect an artifact of enhanced clearance of RBCs with higher antigen levels within each distinct KEL+ RBC population. To examine whether the apparent changes in KEL antigen levels over time simply reflected preferential clearance of RBCs with higher KEL positivity, we compared the clearance of each KEL+ RBC population over time with loss of the KEL antigen. With the exception of KELlo RBCs, very little clearance or antigen loss was observed following transfusion of KEL+ RBCs into recipients immunized with the lowest anti-KEL antibody levels (Figure 4A). In contrast, although each KEL+ RBC population experienced an initial wave of RBC clearance shortly following transfusion into recipients with medium or high anti-KEL antibody levels, loss of detectable KEL antigen continued to occur on each KEL+ RBC population, despite a lack of appreciable changes in RBC clearance over the same period (Figure 4B-C). Changes in KEL antigen levels on each population appeared to be specific because Ter119 levels failed to exhibit a similar change when evaluated in parallel (Figure 4D).
Anti-KEL antibodies induce changes in KEL antigen levels independent of KEL RBC clearance. (A) KELlo (green), KELmed (blue), and KELhi (red) RBC survival was plotted against KEL antigen levels relative to nonimmunized recipients at 10 minutes, and 1, 2, 4, 6, and 24 hours posttransfusion in recipients immunized with 2 µL anti-KEL antibodies (low αKEL). (B) KELlo (green), KELmed (blue), and KELhi (red) RBC survival was plotted against KEL antigen levels relative to nonimmunized recipients at 10 minutes, and 1, 2, 4, 6, and 24 hours posttransfusion in recipients immunized with 6.25 µL anti-KEL antibodies (med αKEL). (C) KELlo (green), KELmed (blue), and KELhi (red) RBC survival was plotted against KEL antigen levels relative to nonimmunized recipients at 10 minutes, and 1, 2, 4, 6, and 24 hours posttransfusion in recipients immunized with 24 µL anti-KEL antibodies (hi αKEL). (D-G) Ter119 levels on KELlo (green), KELmed (blue), and KELhi (red) were evaluated at 10 minutes, and 1, 2, 4, 6, and 24 hours posttransfusion by flow cytometric examination. Error bars represent mean ± SEM. n = 5-7 mice per group. Each panel represents data reproduced 3 times.
Anti-KEL antibodies induce changes in KEL antigen levels independent of KEL RBC clearance. (A) KELlo (green), KELmed (blue), and KELhi (red) RBC survival was plotted against KEL antigen levels relative to nonimmunized recipients at 10 minutes, and 1, 2, 4, 6, and 24 hours posttransfusion in recipients immunized with 2 µL anti-KEL antibodies (low αKEL). (B) KELlo (green), KELmed (blue), and KELhi (red) RBC survival was plotted against KEL antigen levels relative to nonimmunized recipients at 10 minutes, and 1, 2, 4, 6, and 24 hours posttransfusion in recipients immunized with 6.25 µL anti-KEL antibodies (med αKEL). (C) KELlo (green), KELmed (blue), and KELhi (red) RBC survival was plotted against KEL antigen levels relative to nonimmunized recipients at 10 minutes, and 1, 2, 4, 6, and 24 hours posttransfusion in recipients immunized with 24 µL anti-KEL antibodies (hi αKEL). (D-G) Ter119 levels on KELlo (green), KELmed (blue), and KELhi (red) were evaluated at 10 minutes, and 1, 2, 4, 6, and 24 hours posttransfusion by flow cytometric examination. Error bars represent mean ± SEM. n = 5-7 mice per group. Each panel represents data reproduced 3 times.
Because detectable KEL antigen levels quickly change following transfusion into recipients with higher anti-KEL antibody levels, it is difficult to determine whether the initial levels of bound antibody are indeed distinct on each KEL+ RBC population following transfusion into each uniquely anti-KEL immunized recipient. This is apparent for KELhi and KELmed RBCs, where the initial antibody levels appeared to be similar despite differences in pretransfusion antigen levels, especially following transfusion into recipients with the highest anti-KEL antibody levels (Figure 3A). Because incompatible RBC transfusion can result in complement activation, RBC complement deposition is often used clinically as surrogate for antibody activity. As such, complement on the RBC surface may provide some insight into the initial level of antibody activity regardless of detectable antibody binding.39 To test this, we examined complement levels on the surface of each KEL+ RBC population following incompatible transfusion by examining both levels of C3/C3b/iC3b and total C3 levels using monoclonal antibodies that only detects epitopes present in C3/C3b/iC3b or all forms of C3 (total C3), respectively. Using this approach, detection of C3/C3b/iC3b suggests the presence of complement species that may still be present before degradation to C3d (C3d is detected within total C3) as done previously.46,56,57 Levels of C3/C3b/iC3b could not be detected on any KEL+ RBC populations within 10 minutes of transfusion into nonimmunized recipients or recipients with low anti-KEL antibody levels (Figure 5A-B). In contrast, C3/C3b/iC3b could be detected on all KEL+ RBC populations transfused into medium or high anti-KEL antibody immunized recipients, with the levels of C3/C3b/iC3b correlating with the initial KEL antigen and anti-KEL antibody levels (Figure 5C-D). However, the levels of C3/C3b/iC3b detected on each KEL RBC population quickly declined, becoming virtually undetectable within 1 hour following transfusion (Figure 5C-D). This outcome suggests potential C3 degradation. Consistent with this, although C3/C3b/iC3b rapidly declined, total C3 levels could be detected on each KEL RBC surface following transfusion into anti-KEL immunized, but not nonimmunized recipients (Figure 5E-H). The highest C3 levels were observed on KEL+ RBCs exposed to recipients with the highest anti-KEL antibodies levels, although these levels gradually declined over time (Figure 5H). In contrast, although less C3/C3b/iC3b could be detected on the surface of each KEL+ RBC population following transfusion into recipients with medium or low anti-KEL antibody levels (Figure 5B-C), each KEL+ RBC population exhibited a gradual accumulation of total C3 over time (Figure 5F-G). Differences in total C3 levels between groups suggest that the kinetics, magnitude, and overall activity of complement deposition may be influenced by antigen density and antibody levels following an incompatible transfusion.
Anti-KEL antibodies induce complement fixation on KELlo, KELmed, and KELhiRBCs. (A-D) KELlo (green), KELmed (blue), and KELhi (red) RBCs were examined for active complement components (C3/C3b/iC3b) deposition by flow cytometry at 10 minutes, and 1, 2, 4, 6, and 24 hours posttransfusion into low, medium (med), or high (hi) αKEL immunized recipients. (E-H) KELlo (green), KELmed (blue), and KELhi (red) RBCs were examined for total complement component 3 (C3) deposition by flow cytometry at 10 minutes, and 1, 2, 4, 6, and 24 hours posttransfusion into low, medium (med), or high (hi) αKEL immunized recipients. *Significance indicated vs value at 10 minutes posttransfusion. P < .05. Error bars represent mean ± SEM. n = 5-7 mice per group. Each panel represents data reproduced 3 times.
Anti-KEL antibodies induce complement fixation on KELlo, KELmed, and KELhiRBCs. (A-D) KELlo (green), KELmed (blue), and KELhi (red) RBCs were examined for active complement components (C3/C3b/iC3b) deposition by flow cytometry at 10 minutes, and 1, 2, 4, 6, and 24 hours posttransfusion into low, medium (med), or high (hi) αKEL immunized recipients. (E-H) KELlo (green), KELmed (blue), and KELhi (red) RBCs were examined for total complement component 3 (C3) deposition by flow cytometry at 10 minutes, and 1, 2, 4, 6, and 24 hours posttransfusion into low, medium (med), or high (hi) αKEL immunized recipients. *Significance indicated vs value at 10 minutes posttransfusion. P < .05. Error bars represent mean ± SEM. n = 5-7 mice per group. Each panel represents data reproduced 3 times.
In addition to RBC clearance, incompatible RBC transfusion can result in an increase in systemic cytokine levels.33 Such changes may be responsible for or at least influence some of the clinical symptoms of rapid RBC removal.38 As a result, we next sought to define whether the density of the target antigen dictates the overall level of systemic cytokine release observed following transfusion of each KEL+ RBC population. To accomplish this, we examined serum interleukin-6 (IL-6), which has pyrogenic activity and has been previously shown to increase following incompatible transfusion33,62 and interferon-β (IFN-β), a cytokine that can increase in response to viral infection,63 but is less likely to change following incompatible RBC transfusion, as a control. Each cytokine level was examined 2 hours following each transfusion as outlined previously.33,36 Using this approach, transfusion of KELhi RBCs, but unexpectedly not KELmed RBCs, into recipients with high or medium anti-KEL antibodies resulted in increased IL-6, but not IFN-β (Figure 6A-B). Similar to KELmed RBCs, transfusion of KELlo RBCs into immunized recipients, regardless of the level of anti-KEL antibodies, likewise failed to induce detectable changes in IL-6 or IFN-β (Figure 6A-B).
Inflammatory cytokines peak 2 hours posttransfusion. Cytokine analysis using flow cytometry evaluated serological IL-6 (A) and IFN-β (B) levels 2 hours posttransfusion with KELlo (green), KELmed (blue), or KELhi (red) RBCs into low (lo), medium (med), or high (hi) αKEL immunized mice. Baseline cytokine levels are represented by the hashed horizontal line in each graph. *IL-6 levels between KELhi RBC and KELmed or KELlo RBC transfusion, P < .01. Error bars represent mean ± SEM. n = 5-7 mice per group. Each panel represents data reproduced 2 times.
Inflammatory cytokines peak 2 hours posttransfusion. Cytokine analysis using flow cytometry evaluated serological IL-6 (A) and IFN-β (B) levels 2 hours posttransfusion with KELlo (green), KELmed (blue), or KELhi (red) RBCs into low (lo), medium (med), or high (hi) αKEL immunized mice. Baseline cytokine levels are represented by the hashed horizontal line in each graph. *IL-6 levels between KELhi RBC and KELmed or KELlo RBC transfusion, P < .01. Error bars represent mean ± SEM. n = 5-7 mice per group. Each panel represents data reproduced 2 times.
Discussion
Incompatible RBC transfusions have long been thought to reflect rare, yet potentially fatal, complications of transfusion. However, recent results suggest that, in certain patient populations, incompatible RBC transfusions may be more common than previously thought.4,6-12,32 Previous studies suggest that donor antigen density may influence the likelihood of RBC clearance following an incompatible RBC transfusion.49 However, whether these correlations observed clinically reflected differences in antigen levels or other underappreciated features of target antigens have remained unknown. Using the KEL model system outlined in the present study, antigen density and concentrations of cognate antibody appear to possibly influence the consequences of an incompatible transfusion.
The ability of antigen density to impact the overall outcome of an incompatible transfusion likely reflects distinct levels of antibody effector system engagement responsible for RBC removal. The degree of Fcγ receptor clustering following engagement of antibody-coated cells may affect the ability of the common γ chain to effectively initiate signaling events that drive erythrophagocytosis of the target cell.39,64-68 Similarly, although engagement of 1 C1q molecule responsible for activating the complement cascade only requires 2 IgG molecules, these 2 IgGs must simultaneously bind C1q and therefore be within sufficient proximity to simultaneously engage C1q.39,42,69,70 Distinct thresholds for the induction of systemic cytokines were also apparent, as only KELhi RBCs induced detectable increases in systemic IL-6; similar increases failed to occur for IFN-β, a cytokine used as a control that is often elevated following viral infection.63 However, although IL-6 was certainly detected following KELhi RBC-incompatible transfusion, whether other systemic cytokines also increase in this setting or following incompatible transfusion of other KEL+ RBC populations in general and at different times posttransfusion remains to be tested. Given the impact of antigen density on RBC clearance, systemic cytokine production, and antigen modulation, these results suggest that unique thresholds may exist for a variety of consequences associated with incompatible transfusion. Future studies will certainly be needed to identify these unique pathways, including the impact of antigen density, on each of these incompatible RBC transfusion outcomes.
Consistent with a role in antigen density dictating antibody effector system engagement, levels of C3/C3b/iC3b on the surface KELhi, KELmed, and KELlo RBCs following transfusion into recipients with the highest level of anti-KEL antibodies correlated with initial antigen levels. No antibody is currently available that is capable of specifically detecting murine C3d. As a result, C3d detection is often indirectly determined by evaluating levels of total C3 in the absence of detectable C3/C3b/iC3b.46,57 In contrast to C3d, C3b and iC3b can serve as ligands for complement receptors capable of facilitating erythrophagocytosis and therefore are thought to retain important activity in complement-mediated RBC removal.39,71 Although very little C3/C3b/iC3b complement could be detected on the surface of any KEL+ population following transfusion into recipients with low levels of anti-KEL antibodies, total C3 levels gradually accumulated over time. These results suggest that a threshold of antigen density may be required to sustain deposition of C3/C3b/iC3b on the RBC surface before additional degradation ensues. Because KEL antigen modulation was observed in the absence of corresponding reductions in bound antibody, loss of KEL antigen in this setting may also reflect a more gradual, complement-mediated process. However, because complement is only a surrogate for antibody activity, which antibody effector systems may be responsible for KEL antigen modulation in this setting remains to be tested. Regardless of the mechanism whereby antigen modulation occurs, when lower antigen densities or antibody levels are present, the kinetics of complement activation and subsequent degradation may not allow sufficient levels of more active forms of complement (C3b/iC3b) to accumulate and therefore facilitate KEL+ RBC clearance following an incompatible transfusion.
Perhaps one of the most intriguing findings of this study is that, in contrast to the impact of antigen density on RBC removal, the threshold required for antigen modulation is distinct from the level of antigen required for RBC removal. When higher levels of antigen are present, sufficient antigen may initially remain on the cell surface to allow antibody effector systems to induce RBC removal. However, when antigen levels are lower, equally efficient antigen loss may prevent antibody effector systems from reaching thresholds required to sustain engagement of antibody effector programs required for RBC removal. The ability of anti-KEL antibodies to remove KEL from the RBC surface irrespective of antigen density is consistent with recent results. Antibody engagement of CD38 on RBCs in patients treated with daratumumab results in the rapid loss of the target antigen,48 possibly explaining why patients on daratumumab retain a positive antibody screen, despite a negative direct antiglobulin test. Similar results were initially observed in model systems targeting the model HEL antigen.34,35 In contrast, engagement of RhD by anti-RhD following immune thrombocytopenic purpura treatment can induce a marked reduction in antigen levels with accompanying RBC clearance.47 Interactions between a given alloantigen and other RBC surface structures may alter the lateral mobility of the antigen and therefore affect the consequence of alloantibody engagement. The orientation of alloantibody binding sites may also influence the overall consequences of antibody binding following an incompatible RBC transfusion.39,42 Such differences may be even more pronounced when carbohydrate alloantigens are an antibody target.42,71 Carbohydrate alloantigens often exist at much higher overall density than protein alloantigens and often reflect relatively mobile glycolipid structures, features that would be predicted to affect the ability of cognate alloantibodies to cluster or otherwise engage these alloantigens.72 Although not examined in this study, distinct features of target antigens, including antigen density, may likewise influence the outcome of other RBC alloantibody-mediated processes, such as hemolytic disease of the fetus and newborn. These results suggest that although antigen loss can occur following engagement of distinct antigens, the overall likelihood of RBC clearance vs antigen loss may differ depending on the target antigen.
Although the KEL model system provides a unique opportunity to examine antigen density as an isolated variable on the outcome of an incompatible transfusion, this study is not without limitations. Although the KELhi, KELmed, and KELlo RBC populations have different overall levels of the KEL antigen, and the consequences of transfusing each population into anti-KEL antibody-immunized recipients appear to be distinct, subpopulations within each KELhi and KELmed RBC populations were apparent. This finding makes it difficult to determine the impact that more defined and uniform densities of target antigens may have on incompatible RBC transfusions. Furthermore, as noted previously, antigen thresholds required to engage antibody effector systems may differ depending on the antigen involved. It is also not known whether the overall consequences of anti-KEL antibody engagement, including RBC removal and the rate of antigen loss in this model, accurately reflect RBC removal clinically. KEL antigen numbers in humans have been previously reported to range from 3500 to 18 000 per cell.73 Although differences in human vs mouse KEL RBC antigen numbers used in this study may exist, particularly for KELlo RBCs, murine RBCs are smaller and therefore have lower surface area than human RBCs,74 reducing the ability to directly compare these numbers. Future studies examining a larger patient population will be required to obtain a more comprehensive view of KEL antigen density distribution; for the purposes of this study, KELhi, KELmed, and KELlo were simply used to denote differences in the RBC populations used in this model. As a result, direct extrapolation of these findings to all antigens or even the KEL antigen in patients should be undertaken with caution. However, because recent studies suggest that antigen loss can occur following infusion of antibodies that engage RBC antigens in patients and RBC removal appears to correlate with the initial levels of the target antigen,47,48 the general concept that antigen levels may influence incompatible RBC transfusion outcomes is likely relevant in at least in some incompatible transfusions. Furthermore, antigen density can be influenced by the underlying genetics of blood donors, with individuals homozygous and heterozygous for a given antigen often possessing different levels of that antigen on the RBC surface.75,76 This raises the possibility that antigen levels on donor RBCs may be an important variable that influences transfusion outcomes clinically. However, because testing this formally in patients is often unethical and certainly beyond the scope of the present study, only corollaries with limited clinical data can be entertained.
Taken together, the results of this study demonstrate that, in this model system, antigen density can dictate the consequence of antibody-mediated RBC clearance. In doing so, these findings suggest that RBC clearance and loss of target antigen are processes that possess distinct thresholds for activation. Understanding factors that could possibly separate antigen loss from RBC clearance may prove useful in deliberately favoring antigen loss over clearance, thereby protecting otherwise incompatible RBCs from rapid removal. Future studies will be needed to carefully dissect the pathways that govern both antigen loss and antibody-mediated RBC clearance in an effort to facilitate antigen loss over clearance as a novel approach to intervene in patients at-risk for antibody-mediated RBC removal.
Send data sharing requests via e-mail to the corresponding authors, John D. Roback (jroback@emory.edu) or Sean R. Stowell (srstowell@bwh.harvard.edu).
Acknowledgments
The authors appreciate Dan Kalman for careful review and discussion.
This work was supported in part by National Institutes of Health, National Heart, Lung, and Blood Institute grants R01 HL138656 and P01 HL132819 (S.R.S.) and R01 HL135575 (S.R.S. and J.D.R.)
Authorship
Contribution: C.M.A., J.W.L.A., H.V., J.Y., J.D.R., and S.R.S. designed research, analyzed data, and contributed to the writing of the manuscript, which was additionally commented on by R.P.J., K.G.-P., S.C., P.Z., C.M., J.R., R.F., and C.D.J.; H.V., R.P.J., K.G.-P., S.C., P.Z., and C.M. provided critical reagents, assisted in experimental approaches, and aided in data analysis; and R.F. and C.D.J. were essential to developing the overall concept, the interpretation of data, and likewise aided in the design of additional experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John D. Roback, Center for Transfusion and Cellular Therapies, Department of Pathology and Laboratory Medicine, Emory University School of Medicine, D-655, Emory University Hospital, 1364 Clifton Rd NE, Atlanta, GA 30322; e-mail jroback@emory.edu; and Sean R. Stowell, Joint Program in Transfusion Medicine, Brigham and Women’s Hospital, Harvard Medical School, 630D New Research Building, 77 Avenue Louis Pasteur, Boston, MA 02115; e-mail: srstowell@bwh.harvard.edu.
References
Author notes
C.M.A. and J.W.L.A. contributed equally to this work.
The full-text version of this article contains a data supplement.