Key Points
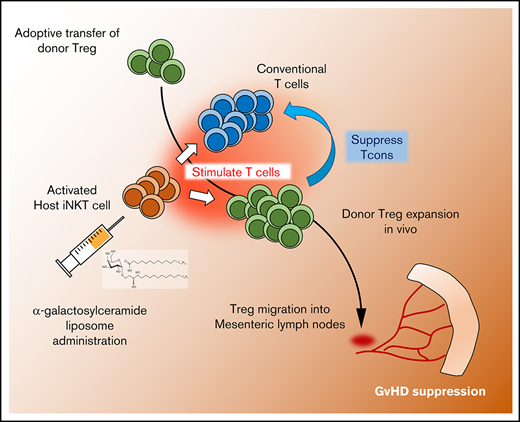
NKT cell activation by α-GalCer-lipo enhanced in vivo expansion of Tregs after adoptive transfer in murine GVHD model.
Injection of α-GalCer-lipo and small numbers of Tregs synergistically suppressed GVHD in a host NKT cell–dependent manner.
Cellular therapy with regulatory T cells (Tregs) has shown promising results for suppressing graft-versus-host disease (GVHD) while preserving graft vs tumor effects in animal models and phase 1/2 clinical trials. However, a paucity of Tregs in the peripheral blood makes it difficult to acquire sufficient numbers of cells and hampers further clinical application. Invariant natural killer T (iNKT) cells constitute another compartment of regulatory cells that ameliorate GVHD through activation of Tregs after their own activation with α-galactosylceramide (α-GalCer) or adoptive transfer. We demonstrate here that a single administration of α-GalCer liposome (α-GalCer-lipo) enhanced the in vivo expansion of Tregs after adoptive transfer in a murine GVHD model and improved therapeutic efficacy of Treg therapy even after injection of otherwise suboptimal cell numbers. Host iNKT cells rather than donor iNKT cells were required for GVHD suppression because the survival benefit of α-GalCer-lipo administration was not shown in the transplantation of cells from wild-type (WT) C57BL/6 mice into Jα18−/− iNKT cell–deficient BALB/c mice, whereas it was observed from Jα18−/− C57BL/6 donor mice into WT BALB/c recipient mice. The combination of iNKT cell activation and Treg adoptive therapy may make Treg therapy more feasible and safer by enhancing the efficacy and reducing the number of Tregs required.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is a well-established treatment for patients with hematological malignancies. The most common life-threatening complication in HCT is graft-versus-host disease (GVHD) that is driven by immunocompetent T cells in the graft-recognizing host tissues, resulting in organ damage and infection. The benefits of transplantation include the induction of a graft-versus-tumor effect by donor immune cells, which has been shown to be preserved in animals and patients receiving regulatory T cell (Treg)–based therapy.1,2 Recent studies have shown that the adoptive transfer of Tregs potently suppresses acute and chronic GVHD in preclinical studies and clinical trials.3 However, clinical translation of this strategy is hindered by the low frequency of Tregs in the peripheral blood. Although several approaches have been attempted to selectively stimulate Tregs by ex vivo expansion or after in vivo transfer,4-7 clinical translation remains challenging because of the lack of specific targeting of Tregs and the technical challenges and cost of such an approach.
Invariant natural killer T (iNKT) cells are another subset of T lymphocytes that have also been shown to potently suppress GVHD. iNKT cells are characterized by the expression of some NK cell markers as well as an invariant T-cell receptor (iTCR) that recognizes glycolipid antigens presented by the nonpolymorphic major histocompatibility complex (MHC) class 1–like molecule CD1d.8 Before the development of antibodies targeting the iTCR, Zeng et al9 identified specific T-cell subsets in bone marrow grafts that coexpressed NK and T-cell markers and suppressed GVHD in an interleukin-4 (IL-4)–dependent manner. Consistently, in more recent clinical studies, a negative correlation between iNKT cell number in the graft and GVHD incidence has been reported.10,11 In animal models, Pillai et al12 demonstrated that iNKT cells played a pivotal role in the amelioration of GVHD by interacting with Tregs in a total lymphoid irradiation model. Our group has also reported that adoptive transfer of donor- or third party–derived iNKT cells facilitated Treg expansion and suppressed acute and chronic GVHD.13-15 These data collectively suggest that iNKT cell targeting might improve Treg potential for GVHD suppression.
α-galactosylceramide (α-GalCer) is synthetic ligand that selectively binds to iTCR expressed on iNKT cells.16 Upon stimulation by α-GalCer, iNKT cells quickly secrete both type 1 helper T (Th1) cytokines and Th2 cytokines.17 Hashimoto et al18 reported that α-GalCer administration ameliorated GVHD in an IL-4–dependent manner, indicating that Th2 polarization by iNKT cell stimulation resulted in GVHD prevention. Liposomal formulation of α-GalCer (α-GalCer-lipo) was reported to be capable of inducing Tregs through iNKT cell activation in vivo.19 Duramad et al20 used α-GalCer-lipo with the goal of suppressing acute GVHD in lethally irradiated mice. A single administration of α-GalCer-lipo induced preferential expansion of Tregs from the donor-derived T-cell inoculum (without specific Treg adoptive transfer) and resulted in GVHD prevention. In contrast, α-GalCer-lipo monotherapy induced interferon-γ (IFN-γ) production from NK cells and resulted in bone marrow graft rejection in sublethally irradiated recipient mice,21,22 indicating the potential risks of α-GalCer related to Th1 cytokine production. Initial clinical observations have been consistent with the murine models, suggesting that α-GalCer-lipo treatment may be an effective strategy for GVHD prevention.23
In the current study, we explored the combination of α-GalCer-lipo administration and adoptive transfer of Tregs for GVHD prevention to evaluate the synergy and enhancement of each treatment strategy. We found that α-GalCer-lipo administration facilitated the expansion and persistence of adoptively transferred Tregs in vivo. The combination therapy resulted in significant reduction in GVHD morbidity and mortality, even when using a suboptimal number of Tregs.
Material and methods
Animals
Eight- to 12-week-old wild-type (WT) BALB/cJ and C57BL/6 mice were purchased from Jackson Laboratory. To obtain highly purified Foxp3-expressing Tregs, we used C57BL/6 Foxp3GFP-DTR mice (B6-Foxp3GFP:H2b, CD45.2+) that were provided by Alexander Rudensky (Memorial Sloan Kettering Cancer Center, New York, NY) as the source of Tregs. C57BL/6–albino background, Foxp3.luciferase-DTR-4 mice (B6-Foxp3GFP/Luc:H2b, CD45.2+), in which expression of GFP/luciferase is controlled by the Foxp3 promoter, were provided by Gunther Hammerling (Heidelberg, Germany).24 BALB/c-background (H2d) and C57BL/6 (H2b) Já18−/− mice were purchased from Jackson Laboratory and bred in the Department of Comparative Medicine, Stanford University. Animal protocols were approved by the Institutional Animal Care and Use Committee of Stanford University.
Reagents
α-GalCer-lipo was kindly provided by REGiMMUNE (Tokyo, Japan). Liposome that does not contain α-GalCer was used for control (Ctrl-lipo). Liposomes were diluted with phosphate-buffered saline (PBS; Life Technologies) for tail vein injection at a dose 10 μg/kg of α-GalCer.
Treg sorting
Single-cell suspensions were obtained from cervical, brachial, axillary, inguinal, and mesenteric lymph nodes (mLNs) and spleens of B6-Foxp3GFP mice or B6-Foxp3GFP/Luc mice. After Fc block (Miltenyi), cells were incubated with Pacific Blue–conjugated anti-CD4, allophycocyanin-conjugated anti-CD8a, and phycoerythrin (PE)-conjugated anti-CD25 (all BioLegend) and the LIVE/DEAD Fixable Aqua Dead Cell Stain Kit (Thermo Fisher Scientific). After washing, cells were incubated with anti-PE MicroBeads (Miltenyi), and PE+ cells were enriched by magnet column isolation. Thereafter, CD4+CD8−CD25hiFoxp3GFP+ live cells were purified by fluorescence-activated cell sorting using the Aria II (BD Biosciences).
Allogeneic BM transplantation
WT or Jα18−/− BALB/c recipient mice (H2d) were treated with total-body irradiation consisting of 2 doses of 4.4 Gy. Bone marrow (BM) cells were isolated from femurs and tibias of WT or Jα18−/− C57BL/6 donor mice (H2b) and were incubated with mixture of CD4 and CD8 MicroBeads (Miltenyi). T-cell depletion (TCD) was performed by magnetic separation with an LS column (Miltenyi). Conventional T cells (Tcons) were isolated from a single-cell suspension obtained from spleen of WT or Jα18−/− C57BL/6 donor mice (H2b) by using a T cell–negative isolation kit (Stemcell); 5.0 × 106 TCD BM cells together with 1.0 × 106 Tcons and 1.0 × 105 purified Tregs were coinjected via tail vein followed by Ctrl- or α-GalCer-lipo administration. GVHD score was assessed as described previously.25 Briefly, weight, fur, skin, activity, and posture were evaluated, and a score of 0 to 2 was assigned to each group, resulting in a maximal GVHD score of 10. We euthanized mice that developed GVHD score >7.
Cytokine analysis
Serum samples were collected from tail veins of the recipient mice at 2 hours and 10 days after transplantation. All samples were analyzed for cytokine concentration through multiplex assay (Luminex; Life Technologies, Logan, UT).
Flow cytometry
Recipient mice were euthanized 10 days after BM transplantation (BMT). Spleen, liver, and LNs were recovered and processed into single-cell suspensions. Samples were incubated with Fc block (Miltenyi) followed by staining with an antibody cocktail purchased from BioLegend: CD4 (GK1.5), CD8a (53-6.7), CD45.1 (A20), CD45.2 (104), CD25 (PC61.5), CD44 (IM7), CD62L (MEL-14), and ICOS (C398.4A). The LIVE/DEAD Fixable Aqua Dead Cell Stain Kit (Thermo Fisher Scientific) was used to detect dead cells. Cells were fixed and permeabilized with a Foxp3 staining kit (eBioscience), and intracellular staining with Ki67 (16A8) and Foxp3 (FJK-16s) was performed. Data were acquired by LSRII flow cytometer (BD Biosciences) and analyzed with FlowJo software (BD Biosciences).
BLI
Bioluminescent imaging (BLI) was performed as described previously26 on days 4, 7, 10, 14, 21, and 28 after BMT. Briefly, D-Luciferin Firefly (Biosynth) was injected intraperitoneally 10 minutes before acquisition with Ami Imager, and images were analyzed with Aura software (Spectral Instruments Imaging, Tucson, AZ). In some experiments, mice were euthanized after BLI, and organs were aligned and the image captured.
Immunohistochemistry
Mouse tissues were harvested and fixed in 4% paraformaldehyde (in PBS [pH, 7.4]; Electron Microscopy Services) overnight at 4°C, embedded, and cryosectioned as 10-μm thin slices. Tissues were permeabilized with 0.5% TritonX-100 (in PBS) for 10 minutes at room temperature and then blocked with 5% normal goat serum, 0.1% TritonX-100, and 1% bovine serum albumin in PBS (pH, 7.4) for 30 minutes at room temperature, followed by incubation with primary antibodies in the same blocking solution overnight at 4°C. The next day, tissues were washed with PBS three times at 5-minute intervals and then incubated with secondary antibodies diluted in PBS for 2 hours at room temperature. After washing with PBS three times for 5 minutes each, tissues were mounted in antifade fluorescent mounting reagent with 4′,6-diamidino-2-phenylindole (Thermal Fisher #P36963) and coverslipped. The following primary antibodies were used: rabbit anti-GFP (1:1000; Abcam #abs290) and rat anti-CD4 (1:100; BD Biosciences). Alexa Fluor secondary antibodies (488 or 594; 1:200; Invitrogen) were also used for visualization. Representative fluorescent images from sections were captured using Keyence BZ-X810 (Keyence).
Statistics
All statistics were calculated using GraphPad Prism 8 (GraphPad software). Differences in animal survival (Kaplan-Meier survival curves) were analyzed with the log-rank test. All other comparisons were performed with the Student t test for 2-group comparison and with ordinally 1-way analysis of variance for multiple comparisons. P value <.05 was considered statistically significant.
Results
α-GalCer-lipo administration alone does not affect GVHD
We investigated the effect of α-GalCer-lipo in a murine model of acute GVHD without Treg transfer. As shown in Figure 1A-B, mice that received Tcons showed rapid body weight loss and increase in GVHD score after BMT. In the Ctrl-lipo group, 2 of 20 mice died by day 14 (Figure 1C green line). In contrast, 9 of 20 mice in the α-GalCer-lipo group died by day 14, suggesting that α-GalCer-lipo administration may have exacerbated acute toxicity (yellow line; 20% to 60% incident rate of acute toxicity in each experiment). To address the cause of the acute toxicity, we measured serum cytokine levels at 2 hours and 10 days after α-GalCer-lipo injection (supplemental Figure 1A). The surge of a variety of cytokines was observed in α-GalCer-lipo–treated mice (IFN-γ, IL-2, granulocyte-macrophage colony-stimulating factor, tumor necrosis factor α, IL-6, and IL-4), suggesting that the acute lethal toxicity might have been caused by cytokine release syndrome. To exclude the impact of acute toxicity, we compared survival among the mice that survived beyond day 14 (supplemental Figure 2). In this analysis, α-GalCer-lipo injection significantly improved the survival rate compared with the Ctrl-lipo group (P = .0003). The serum cytokine level of proinflammatory cytokines was reduced in α-GalCer-lipo group at day 10 after BMT (supplemental Figure 1B). These data collectively suggest that α-GalCer-lipo treatment exerted an anti-inflammatory effect and prevented GVHD development after recovery from the initial cytokine storm. However, the overall survival rate was not significantly different between Ctrl-lipo and α-GalCer-lipo groups in this model (P = .16).
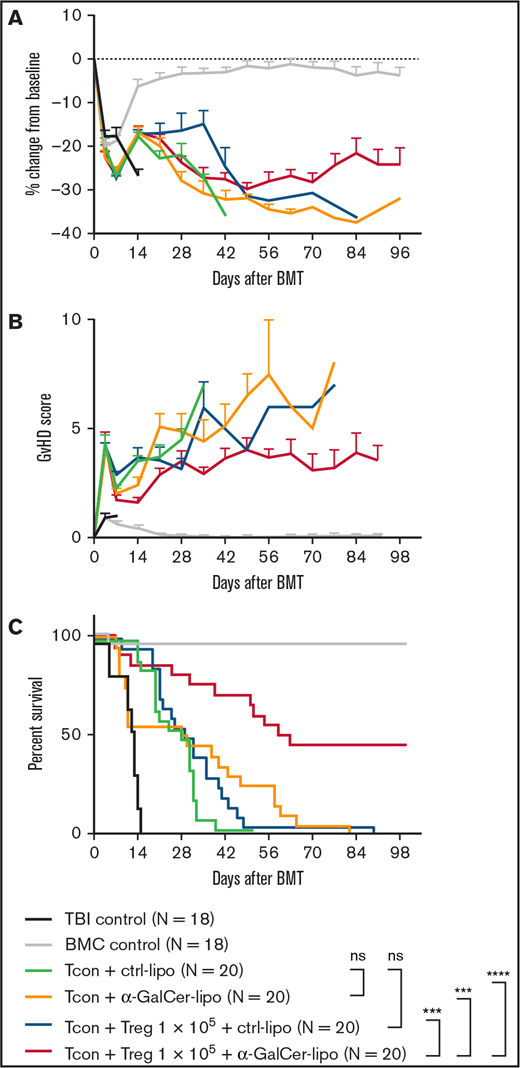
Combination of α-GalCer-lipo administration plus low-dose Tregs suppresses GVHD. BALB/c recipient mice undergoing irradiation at 8.8 Gy received 5.0 × 106 T cell–depleted BM cells (BMCs) and 1.0 × 106 negatively isolated Tcons obtained from C57BL/6 donor mice. CD4+Foxp3GFP+ Tregs were isolated from C57BL/6 background Foxp3GFP transgenic mice and injected together with Tcons at 1:10 Treg/Tcon ratio. Immediately after cell infusion, Ctrl-lipo or α-GalCer-lipo was injected via tail vein (10 μg/kg). (A) Mean ± standard error of the mean (SEM) of percentage change in body weight from baseline (day before BMT). (B) Mean ± SEM of GVHD score at indicated time points. (C) Survival curve of the recipients in each group. P values calculated by log-rank test between 2 indicated groups. Pooled data of 4 independent experiments are shown. ***P < .001, ****P < .0001. ns, not significant; TBI, total-body irradiation alone.
Combination of α-GalCer-lipo administration plus low-dose Tregs suppresses GVHD. BALB/c recipient mice undergoing irradiation at 8.8 Gy received 5.0 × 106 T cell–depleted BM cells (BMCs) and 1.0 × 106 negatively isolated Tcons obtained from C57BL/6 donor mice. CD4+Foxp3GFP+ Tregs were isolated from C57BL/6 background Foxp3GFP transgenic mice and injected together with Tcons at 1:10 Treg/Tcon ratio. Immediately after cell infusion, Ctrl-lipo or α-GalCer-lipo was injected via tail vein (10 μg/kg). (A) Mean ± standard error of the mean (SEM) of percentage change in body weight from baseline (day before BMT). (B) Mean ± SEM of GVHD score at indicated time points. (C) Survival curve of the recipients in each group. P values calculated by log-rank test between 2 indicated groups. Pooled data of 4 independent experiments are shown. ***P < .001, ****P < .0001. ns, not significant; TBI, total-body irradiation alone.
α-GalCer-lipo administration plus suboptimal number of Tregs suppresses GVHD
Next, we evaluated the impact of α-GalCer-lipo administration in addition to Treg transfer (Figure 1A-C). CD4+CD25+Foxp3GFP+ Tregs were isolated from H2b+Foxp3GFP+ (same background as BM/Tcon donor mice; supplemental Figure 3). Isolated donor Tregs were transferred together with Tcons at 1:10 Treg/Tcon ratio. At this suboptimal dose of Tregs, the survival rate of the mice that received Tcons plus Tregs plus Ctrl-lipo (blue line) was not significantly different from that of mice that received Tcons plus Ctrl-lipo without Tregs (green line; P = .17; Figure 1C). Mice that received Tcons plus Tregs plus α-GalCer-lipo (red line) showed significantly prolonged survival compared with mice that received Ctrl-lipo alone (green line; P < .0001), α-GalCer-lipo alone (yellow line; P = .0001), or Tcons plus Tregs plus Ctrl-lipo (blue line; P = .0001). Because the serum cytokine levels at 2 hours after α-GalCer-lipo injection were not different between the no Treg and Treg transfer groups (supplemental Figure 1), it seemed that Tregs did not directly affect iNKT cell activation. However, the early acute toxicity that was observed in the mice treated with α-GalCer-lipo alone was lower in animals treated with Tregs plus α-GalCer-lipo (3 of 20). Furthermore, the survival benefit of α-GalCer-lipo plus Treg combination therapy was also shown when the mice that died before day 14 were excluded (supplemental Figure 2; vs. Ctrl-lipo alone, P < .0001; vs. α-GalCer-lipo alone, P = .006; and vs. Tregs plus Ctrl-lipo, P < .0001). These data indicate that Treg transfer suppresses the toxic effect of α-GalCer-lipo as well as enhances the GVHD preventive effect by synergizing with α-GalCer-lipo administration. This synergistic effect enabled the suppression of GVHD when the Treg/Tcon ratio was 1:20 (supplemental Figure 4).
α-GalCer-lipo administration increases both Tcon-derived and adoptively transferred Tregs
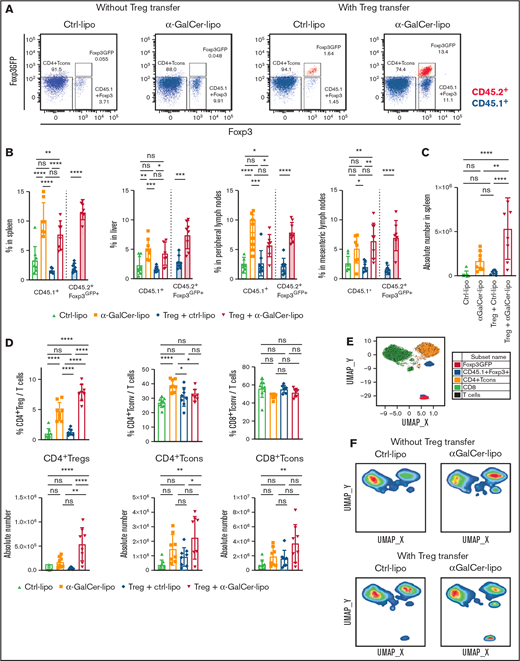
To address the effect of α-GalCer-lipo on Tregs, we analyzed Treg proportions within CD4+ T cells in the spleen, liver, and peripheral LNs (pLNs) and mesenteric LNs (mLNs) of the recipient mice by flow cytometry 10 days after BMT (Figure 2A-B). We used CD45.1+ C57BL/6 mice as Tcon donors to distinguish Tcon-derived CD45.1+Foxp3+ Tregs from CD45.2+Foxp3GFP+ adoptively transferred Tregs. As shown in Figure 2A, the CD4+Foxp3+ cell population can be divided into CD45.1+Foxp3+ Tregs from Tcon inoculum (blue) and CD45.2+Foxp3GFP+ adoptively transferred Tregs (red). Notably, all the CD45.2+ transferred Tregs retained Foxp3GFP expression after in vivo proliferation. In the animals without Treg transfer, the α-GalCer-lipo group had a significantly higher proportion of Tcon-derived Tregs compared with the Ctrl-lipo group in the spleen (P < .0001), liver (P = .004), and pLNs (P < .0001). Similar trends were observed in the mLNs, although the difference did not reach statistical significance (P = .07). As in animals without Treg transfer, mice treated with Tregs plus α-GalCer-lipo showed a higher proportion of Tcon-derived Tregs than mice receiving Tregs plus Ctrl-lipo (spleen, P < .0001; liver, P = .01; pLNs, P = .04; and mLNs, P = .001). In addition, those mice also had a higher proportion of adoptively transferred Tregs as compared with mice receiving Tregs with Ctrl-lipo (spleen, P < .0001; liver, P = .0002; pLNs, P < .0001; and mLNs, P < .0001). Accordingly, the number of total splenic Tregs in the Tregs plus α-GalCer-lipo group was 14.5-fold higher than that in the Tregs plus Ctrl-lipo group (Figure 2C; 5.3 × 105 ± 3.4 × 105 in Tregs plus α-GalCer-lipo group [red] and 0.4 × 105 ± 0.3 × 105 in Tregs plus Ctrl-lipo group [blue]; P < .0001), whereas the difference between Ctrl-lipo and α-GalCer-lipo was not statistically significant when Tregs were not transferred (1.7 × 105 ± 1.1 × 105 in α-GalCer-lipo group [brown] and 0.2 × 105 ± 0.3 × 105 in Ctrl-lipo group [green]; P = .3). These data indicate that Treg transfer enhances the effect of α-GalCer-lipo by increasing the total number of Tregs.
α-GalCer-lipo administration promotes both Tcon-derived Tregs and adoptively transferred Treg proliferation. WT BALB/c mice (CD45.2+H2d+) received BM cell transplants from WT C57BL/6 donor mice (CD45.2+H2b+) and transferred Tcons from CD45.1+ C57BL/6 and Tregs from CD45.2+Foxp3GFP+ C57BL/6 donor mice. Spleen, liver, pLNs, and mLNs were recovered from the recipient mice on day 10 after BMT. A single-cell suspension was analyzed by flow cytometry. (A) Representative plots of CD4+ gated splenocytes. x-axis shows Foxp3 expression detected by intracellular staining; y-axis shows Foxp3GFP expression. CD45.2 gated cells are shown in red; CD45.1 gated cells are shown in blue. Lower left box indicates Tcons; lower right box indicates Tcon-derived Tregs; upper right box indicates transferred Tregs. (B) Mean ± standard deviation (SD) of the proportion of Foxp3+/total CD4+ T cells in the indicated organ. Open bar indicates CD45.1+ Tcon-derived Tregs; filled bar indicates CD45.2+Foxp3GFP+ transferred Tregs. (C) Absolute number of total CD4+Foxp3+ Tregs in the spleen. (D) Proportion among whole T cells (above) and absolute numbers (bottom) of CD4+ Tregs (including Tcon derived and transferred), CD4+Foxp3− Tcons, and CD8+ Tcons in the spleen. Mean ± SD from pooled data of 2 independent experiments. P values calculated by Student t test for 2-group comparison and ordinary 1-way analysis of variance with Holm-Sidak correction. (E) Uniform manifold approximation and projection (UMAP) plot for T cells from concatenated data set of 1 representative experiment. CD4+Foxp3− Tcons shown in orange; CD8+ Tcons shown in green; CD45.1+ Tcon-derived Tregs shown in blue; Foxp3GFP+ transferred Tregs shown in red. (F) UMAP plots gated on 1 representative mouse in each group. *P < .05, **P < .01, ***P < .001, ****P < .0001.
α-GalCer-lipo administration promotes both Tcon-derived Tregs and adoptively transferred Treg proliferation. WT BALB/c mice (CD45.2+H2d+) received BM cell transplants from WT C57BL/6 donor mice (CD45.2+H2b+) and transferred Tcons from CD45.1+ C57BL/6 and Tregs from CD45.2+Foxp3GFP+ C57BL/6 donor mice. Spleen, liver, pLNs, and mLNs were recovered from the recipient mice on day 10 after BMT. A single-cell suspension was analyzed by flow cytometry. (A) Representative plots of CD4+ gated splenocytes. x-axis shows Foxp3 expression detected by intracellular staining; y-axis shows Foxp3GFP expression. CD45.2 gated cells are shown in red; CD45.1 gated cells are shown in blue. Lower left box indicates Tcons; lower right box indicates Tcon-derived Tregs; upper right box indicates transferred Tregs. (B) Mean ± standard deviation (SD) of the proportion of Foxp3+/total CD4+ T cells in the indicated organ. Open bar indicates CD45.1+ Tcon-derived Tregs; filled bar indicates CD45.2+Foxp3GFP+ transferred Tregs. (C) Absolute number of total CD4+Foxp3+ Tregs in the spleen. (D) Proportion among whole T cells (above) and absolute numbers (bottom) of CD4+ Tregs (including Tcon derived and transferred), CD4+Foxp3− Tcons, and CD8+ Tcons in the spleen. Mean ± SD from pooled data of 2 independent experiments. P values calculated by Student t test for 2-group comparison and ordinary 1-way analysis of variance with Holm-Sidak correction. (E) Uniform manifold approximation and projection (UMAP) plot for T cells from concatenated data set of 1 representative experiment. CD4+Foxp3− Tcons shown in orange; CD8+ Tcons shown in green; CD45.1+ Tcon-derived Tregs shown in blue; Foxp3GFP+ transferred Tregs shown in red. (F) UMAP plots gated on 1 representative mouse in each group. *P < .05, **P < .01, ***P < .001, ****P < .0001.
Treg adoptive transfer plus α-GalCer-lipo administration increases total CD4+ Treg proportion without affecting CD4+ Tcon proportion
To address the impact of α-GalCer-lipo on non-Treg subsets, we compared the proportion of different cells among whole T cells for CD4+Foxp3+ T cells (CD4+ Tregs, including Tcon-derived and transferred Tregs), CD4+Foxp3− Tcons (CD4+ Tcons), and CD8+ T cells (CD8+ Tcons), as well as the absolute number of each subset (Figure 2D). The mice treated with α-GalCer-lipo monotherapy showed an increase in the proportion of both CD4+ Tregs (P < .0001) and CD4+ Tcons (P < .0001), as well as in the absolute number of those cells, although the difference did not reach statistical significance (CD4+ Tregs, P = .2632 and CD4+ Tcons, P = .0840). The results indicate that α-GalCer-lipo administration facilitated CD4+ T-cell reconstitution. Treg transfer alone did not alter Treg number or Treg proportion compared with Ctrl-lipo alone (P = .68), indicating that the transfer of 1 × 105 Tregs alone was not strong enough to modify T-cell subsets. The same number of Treg transfers together with α-GalCer-lipo administration significantly increased the Treg proportion (P < .0001) and Treg number (P < .0001), suggesting that α-GalCer-lipo administration enhanced the impact of Treg transfer. Notably, combination of Tregs plus α-GalCer-lipo did not affect the proportion of CD4+ Tcons (P = .651).
To further assess the impact of α-GalCer-lipo on T cells, we performed an unsupervised analysis of T-cell phenotype using T-cell markers, including activation and differentiation markers (CD25, CD44, CD62L, ICOS, and Ki67), and assessed uniform manifold approximation and projection. The plot identified 4 major clusters representing each T-cell subset: CD8+ Tcons (cluster 1, green), CD4+Foxp3− Tcons (cluster 2, orange), CD45.1+CD4+Foxp3+ Tregs (cluster 3, blue), and CD4+Foxp3GFP+ Tregs (cluster 4, red; Figure 2E; supplemental Figure 5A-D). The proportion of each T-cell cluster in an individual mouse was calculated by gating on sample number (supplemental Figure 5F-G), and the quantification of T-cell clusters is shown in supplemental Figure 5D. Consistent with the results of the population analysis (Figure 2D top), the α-GalCer-lipo alone group revealed a significant increase in CD4+Foxp3− Tcons (cluster 2), whereas the group receiving α-GalCer-lipo plus Tregs did not show an increase in cluster 2 but did show an increase in CD4+Foxp3GFP+ Tregs (cluster 4; Figure 2F; supplemental Figure 5E). Altogether, these data demonstrate that α-GalCer-lipo stimulation facilitated the expansion of transferred Foxp3GFP+ Tregs and skewed the T-cell balance into a Treg-dominant state.
α-GalCer-lipo promotes the in vivo expansion of transferred Tregs
The increase in Foxp3GFP+-transferred Tregs was also observed with earlier time point flow cytometry (day 6; supplemental Figure 6). To trace Treg kinetics and distribution after in vivo transfer, Tregs were obtained from Foxp3GFP+Luc+ C57BL/6 mice that express both GFP and luciferase. Consistent with the experiment shown in Figure 1, mice that received Foxp3GFP+Luc+ Tregs together with α-GalCer-lipo survived significantly longer than those receiving Ctrl-lipo (Figure 3A; P < .0001). Using BLI, photons from transferred Tregs were observed at similar levels in both Ctrl-lipo and α-GalCer-lipo groups by day 7 (Figure 3B-C; P > .99 on day 4; P = .99 on day 7). In the Ctrl-lipo group, the Treg signal decreased after day 14. In contrast, the mice treated with α-GalCer-lipo showed significantly higher total photon flux than those in the Ctr-lipo group on days 14 and 21 (P = .003 and P = .0010, respectively). The signals gradually decreased thereafter, and the difference between the 2 groups eventually disappeared after day 28. The data suggest that a single administration of α-GalCer-lipo promoted the expansion of Tregs and prolonged their survival for 2 to 3 weeks after BMT.
α-GalCer-lipo administration enhances the in vivo expansion of transferred Tregs. Flow-sorted Foxp3GFP+Luc+ cells were injected into recipient mice at 1:10 Treg/Tcon ratio at the time of BMT. Recipient mice were treated with Ctrl-lipo or α-GalCer-lipo. (A) Survival curve. (B) Images of photons from Foxp3GFP+Luc+ cells in 1 representative experiment. White X indicates mice already dead at the analysis. (C) Mean ± standard error of the mean for total flux (photons per second [p/s]) from transferred Foxp3Luc+ cells in indicated groups. Data pooled from 3 independent experiments. **P < .01 calculated by log-rank test between indicated 2 groups (A) or by 2-way analysis of variance between Ctrl-lipo and α-GalCer-lipo at indicated time point (C), ***P < .001 by 2-way analysis of variance between Ctrl-lipo and α-GalCer-lipo at indicated time point. BMC, BM cell; TBI, total-body irradiation.
α-GalCer-lipo administration enhances the in vivo expansion of transferred Tregs. Flow-sorted Foxp3GFP+Luc+ cells were injected into recipient mice at 1:10 Treg/Tcon ratio at the time of BMT. Recipient mice were treated with Ctrl-lipo or α-GalCer-lipo. (A) Survival curve. (B) Images of photons from Foxp3GFP+Luc+ cells in 1 representative experiment. White X indicates mice already dead at the analysis. (C) Mean ± standard error of the mean for total flux (photons per second [p/s]) from transferred Foxp3Luc+ cells in indicated groups. Data pooled from 3 independent experiments. **P < .01 calculated by log-rank test between indicated 2 groups (A) or by 2-way analysis of variance between Ctrl-lipo and α-GalCer-lipo at indicated time point (C), ***P < .001 by 2-way analysis of variance between Ctrl-lipo and α-GalCer-lipo at indicated time point. BMC, BM cell; TBI, total-body irradiation.
Transferred Tregs accumulate in mLNs
Figure 3B demonstrates that the transferred Tregs accumulated in cervical LNs and abdominal space in α-GalCer-lipo–treated mice. To elaborate Treg distribution, we evaluated luciferase activity in the heart, lung, liver, spleen, kidney, and intestines ex vivo (Figure 4A-B). A majority of photons were observed in intestines in both Ctrl-lipo– and α-GalCer-lipo–treated mice, although there was no statistical difference between the 2 treatment groups in the intestines (P = .5), spleen (P = .9), or liver (P = .7). Notably, in α-GalCer-lipo–treated mice, a significant accumulation of Tregs was observed in the mLNs compared with those receiving Ctrl-lipo (P = .006). We performed immunohistochemistry to identify localization of Foxp3GFP+ Tregs in the small intestines and mLNs. Consistent with severe GVHD, Ctrl mice had greater CD4+ T-cell infiltration in the small intestines compared with α-GalCer-lipo–treated mice (supplemental Figure 7). CD4+Foxp3GFP+ Tregs were hardly detected in the intestines in either group. Similarly, mLNs of Ctrl mice also revealed abundant CD4+ T-cell infiltration compared with those of α-GalCer-lipo–treated mice (Figure 4C). In line with the BLI results, α-GalCer-lipo–treated mice showed accumulation of CD4+Foxp3GFP+ Tregs in mLNs that was not observed in Ctrl mice. These data collectively suggest that α-GalCer-lipo treatment affected Treg migration into LNs.
Tregs accumulate in mLNs in α-GalCer-lipo–treated mice. Recipient mice received Foxp3GFP+Luc+ cells with Ctrl-lipo (blue; n = 7) or α-GalCer-lipo (red; n = 7) and were euthanized 5 minutes after luciferase injection on day 10 after BMT. Organs were removed and images were acquired within 15 minutes after luciferase injection. (A) Representative images of ex vivo BLI. Far right box indicates region of interest (ROI) for each organ. BM cell (BMC; gray; n = 6) control without Tcon/Foxp3GFP+Luc+ Treg transfer. (B) Mean ± standard deviation of total flux (photons per second [p/s]) obtained from each ROI. Data pooled from 2 independent experiments. (C) Images of immunofluorescent staining of mLN for CD4 (Alexa Fluor 594; red), Foxp3GFP (Alexa Fluor 488; green), and 4′,6-diamidino-2-phenylindole (DAPI; blue). Left shows low magnification (10×; white bar indicates 100 μm); right shows high magnification of inset region (40×; white bar indicates 20 μm). *P < .05, **P < .01, ***P < .001 between indicated 2 groups calculated by ordinary 1-way analysis of variance with Holm-Sidak correction.
Tregs accumulate in mLNs in α-GalCer-lipo–treated mice. Recipient mice received Foxp3GFP+Luc+ cells with Ctrl-lipo (blue; n = 7) or α-GalCer-lipo (red; n = 7) and were euthanized 5 minutes after luciferase injection on day 10 after BMT. Organs were removed and images were acquired within 15 minutes after luciferase injection. (A) Representative images of ex vivo BLI. Far right box indicates region of interest (ROI) for each organ. BM cell (BMC; gray; n = 6) control without Tcon/Foxp3GFP+Luc+ Treg transfer. (B) Mean ± standard deviation of total flux (photons per second [p/s]) obtained from each ROI. Data pooled from 2 independent experiments. (C) Images of immunofluorescent staining of mLN for CD4 (Alexa Fluor 594; red), Foxp3GFP (Alexa Fluor 488; green), and 4′,6-diamidino-2-phenylindole (DAPI; blue). Left shows low magnification (10×; white bar indicates 100 μm); right shows high magnification of inset region (40×; white bar indicates 20 μm). *P < .05, **P < .01, ***P < .001 between indicated 2 groups calculated by ordinary 1-way analysis of variance with Holm-Sidak correction.
Recipient iNKT cells are required to prevent GVHD after α-GalCer-lipo injection
To determine the origin of the iNKT cells that are required for GVHD prevention, we studied Jα18−/− iNKT cell–deficient mice as both donors (C57BL/6) and recipients (BALB/c). When T cell–depleted BM cells and Tcons were obtained from Jα18−/− C57BL/6 mice and injected into WT BALB/c recipient mice, α-GalCer-lipo plus Treg transfer therapy showed significant improvement in survival (P = .006), indicating that donor iNKT cells were dispensable in this model. In contrast, when we used Jα18−/− BALB/c mice as recipients, α-GalCer-lipo plus Treg transfer therapy no longer demonstrated a survival benefit (Figure 5B; P = .7383). Taken together, these studies demonstrate that α-GalCer-lipo administration enhanced Treg potential for GVHD prevention via host iNKT cell activation.
Host iNKT cells are required for GVHD protection after α-GalCer-lipo treatment. (A) T cell–depleted BM cells (BMCs) and Tcons isolated from C57BL/6 Jα18−/− mice (H2b) were transferred into WT BALB/c mice (H2d) together with purified Foxp3GFP+ Tregs that were obtained from C57BL/6 Foxp3GFP+ mice (H2b). (B) T cell–depleted BMCs and Tcons were obtained from WT C57BL/6 donor mice (H2b) and transferred into BALB/c Jα18−/− mice (H2d) together with C57BL/6 Foxp3GFP+ Tregs (H2b). Ctrl-lipo (blue) or α-GalCer-lipo (red) was injected right after cell transfer. Pooled data from 2 independent experiments. **P < .01 by log-rank test between 2 indicated groups.
Host iNKT cells are required for GVHD protection after α-GalCer-lipo treatment. (A) T cell–depleted BM cells (BMCs) and Tcons isolated from C57BL/6 Jα18−/− mice (H2b) were transferred into WT BALB/c mice (H2d) together with purified Foxp3GFP+ Tregs that were obtained from C57BL/6 Foxp3GFP+ mice (H2b). (B) T cell–depleted BMCs and Tcons were obtained from WT C57BL/6 donor mice (H2b) and transferred into BALB/c Jα18−/− mice (H2d) together with C57BL/6 Foxp3GFP+ Tregs (H2b). Ctrl-lipo (blue) or α-GalCer-lipo (red) was injected right after cell transfer. Pooled data from 2 independent experiments. **P < .01 by log-rank test between 2 indicated groups.
Discussion
iNKT cells possess distinct characteristics as compared with Tcons.27 Whereas Tcons recognize peptides that are presented on polymorphic MHC class 1 molecules, iNKT cells recognize glycolipids presented on CD1d:MHC class 1–like molecules that are monomorphic among species.28 Systemic stimulation of iNKT cells by glycolipid ligands induces rapid cytokine production, including IFN-γ, IL-4, IL-10, IL-17, IL-21, and tumor necrosis factor α, that is able to either promote or suppress cell-mediated immunity. iNKT cells exert antitumor adjuvant effects through activation of NK cells, CD8+ T cells, and maturating dendritic cells.28 In contrast, it has been reported that iNKT cells also play a role in suppression of autoimmune diseases, GVHD, and transplant rejection.15,21,29 The mechanism by which iNKT cells exert such diverse biological functions is not clear, although iNKT cells have tissue-specific and distinct phenotypic subsets, and each subset has different functionality.30,31 Different functions might also be conferred by the glycolipid antigens or antigen-presenting cells.32 In the current study, α-GalCer-lipo alone increased both CD4+ Tregs and CD4+ Tcons from the donor T-cell inoculum, indicating that α-GalCer-lipo monotherapy may elicit both pro- and anti-inflammatory reactions. However, administration of both exogenous Tregs and α-GalCer-lipo primarily resulted in an increase of both endogenous and exogeneous Tregs, whereas it did not affect CD4+ Tcons. These data collectively suggest that iNKT cells and Tregs function synergistically to enhance immune regulatory properties, as evidenced by the suppression of GVHD. It may be possible to enhance the immunosuppressive properties of iNKT cells by adjusting the Treg/Tcon ratio through specific preconditioning, such as total lymphoid irradiation/antithymocyte serum,33 rapamycin,34 or Treg adoptive transfer.
Several studies have reported that higher numbers of iNKT cells in allogeneic donor grafts are associated with reduced acute GVHD,10,11,35 consistent with preclinical studies demonstrating that infusion of donor iNKT cells at low numbers of 50 000 cells per animal results in control of GVHD.13 However, in the current study, low doses of Tregs, which have not been effective in preclinical models, when used in combination with α-GalCer-lipo resulted in effective control of GVHD, dependent on host iNKT cells. Hashimoto et al18 reported that α-GalCer stimulation did not confer GVHD protection when they used iNKT cell–deficient CD1d knockout mice as recipients. However, because CD1d−/− mice do not have antigen-presenting cells that can present α-GalCer, the data cannot address the dispensability of donor iNKT cells. Consistent with our results, Haraguchi et al36 directly showed the dispensability of donor iNKT cells in GVHD prevention in an α-GalCer treatment model by using Jα18 knockout iNKT-deficient mice as donors. They also showed that GVHD was more severe in Jα18−/− host mice compared with WT mice, although α-GalCer treatment was not tested in Jα18−/− host mice. We showed here the negative impact of α-GalCer-lipo treatment in Jα18−/− host mice. The data suggest that host iNKT cells play an important role in GVHD prevention, although it is also possible that the GVHD in Jα18−/− host mice was too aggressive to control. Further assessment is necessary to prove the impact of host iNKT cell deficiency on Treg expansion in future studies. Our group previously showed that iNKT cells isolated from third party donors revealed comparable potential for GVHD suppression by iNKT cells from the BM donor,14 indicating the mechanism by which iNKT cells suppress GVHD is not MHC restricted. It is likely that host iNKT cells played a major role in the current study, because the number of host iNKT cells was much larger than that of donor iNKT cells at the time of α-GalCer-lipo injection.
In contrast to other published reports, we did not observe significant improvement in survival or reduction in GVHD in α-GalCer-lipo monotherapy–treated animals.18,20,36 Previous reports used splenocytes to induce GVHD, whereas we used purified T cells to precisely evaluate the Tcon/Treg ratio. In the current study, we detected high serum cytokine levels in the mice treated with α-GalCer-lipo. Although histopathological examination at day 10 after BMT did not identify evidence of cytokine release syndrome (data not shown), we speculate that early and severe acute toxicity in α-GalCer-lipo monotherapy–treated groups may be related to those proinflammatory cytokines. Because the number of donor T cells was higher in our model compared with the splenocyte model, the cytokine storm after α-GalCer administration could be more severe and exacerbate GVHD mortality. Indeed, when we excluded murine death that occurred early within 14 days after BMT, α-GalCer-lipo monotherapy showed significant survival improvement compared with Ctrl-lipo monotherapy. It has also been reported that B cells that incorporate and present α-GalCer induce IL-10 production in Tregs.19 It is possible that donor-derived non-T cells, such as B cells, might be responsible for this observation.
The toxicity shown in α-GalCer monotherapy must be closely addressed, although initial clinical studies showed this agent was quite tolerable.23 Jing Du et al15 reported prophylactic and therapeutic effects of α-GalCer-lipo in an MHC minor mismatch model, which may be more clinically relevant compared with a fully MHC-mismatched model. In their report, α-GalCer-lipo administration prevented chronic lung damage when administered on days 1 and 14 after BMT. Furthermore, α-GalCer-lipo administration on days 28 and 42 ameliorated lung damage. Duramado et al20 showed rapamycin, a conventional immunosuppressant used in the clinic for GVHD prophylaxis and treatment, synergistically improved the survival benefit of α-GalCer-lipo in an acute GVHD model. These data collectively suggest that the toxicity of α-GalCer-lipo could be mitigated by therapeutic protocols. Indeed, no cytokine release–related complications were observed in a phase I/IIa open-label, multicenter, dose-escalation clinical trial of α-GalCer-lipo (RGI-2001) in patients undergoing allogeneic HCT with standard immunosuppressants (registered at www.clinicaltrials.gov as #NCT01379209). In this study, 28% of patients (8 of 29) showed markedly increased number of Tregs after RGI-2001 administration.23 The incidence of grade 2 to 4 GVHD was 12.5%, and no grade 3 to 4 GVHD events were observed in the patients who responded to RGI-2001 administration. An open-label, multicenter, single-arm study to evaluate RGI-2001 in combination with standard-of-care treatment for GVHD prevention is ongoing (registered at www.clinicaltrials.gov as #NCT04014790). We also confirmed feasibility of purified Treg therapy for GVHD prophylaxis in a single-center open phase I/II study (registered at www.clinicaltrials.gov as #NCT01660607)37; this is now being evaluated in a multi-institutional phase II study with Tregs and single-agent immunosuppressive prophylaxis. Our preclinical data suggest that the addition of α-GalCer therapy may improve the clinical effect of Treg therapy for GVHD prevention.
Acknowledgments
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grant P01 HL075462 (R.S.N.) and National Center for Research Resources SIG S10RR027431-01 (Stanford Shared FACS Facility), Japan Society for the Promotion of Science KAKENHI (Grants-in-Aid for Scientific Research) grant 15KK0355 (T.H.), the Geneva University Hospitals Fellowship (F.S.), grant BIL KLS 3806-02-2016 from the Swiss Cancer League (F.S.), and the Fondation de Bienfaisance Valeria Rossi di Montelera Eugenio Litta Fellowship (F.S.).
Authorship
Contribution: T.H. conceived and designed experiments and conducted the experiments with help from P.-Y.L., F.S., K.M.-B., M.T., M.M., and J.B.; R.S.N. helped with critical advice and discussion; and T.H. and R.S.N. wrote the manuscript, with contributions from all authors.
Conflict-of-interest disclosure: M.T. is employed by Allogene Therapeutics and holds stock in the company. The remaining authors declare no competing financial interests.
Correspondence: Robert S. Negrin, Center for Clinical Sciences Research, 269 Campus Dr, Room 2205, Stanford, CA 94305; e-mail: negrs@stanford.edu.
References
Author notes
The full-text version of this article contains a data supplement.
For original data, please contact negrs@stanford.edu.

![α-GalCer-lipo administration enhances the in vivo expansion of transferred Tregs. Flow-sorted Foxp3GFP+Luc+ cells were injected into recipient mice at 1:10 Treg/Tcon ratio at the time of BMT. Recipient mice were treated with Ctrl-lipo or α-GalCer-lipo. (A) Survival curve. (B) Images of photons from Foxp3GFP+Luc+ cells in 1 representative experiment. White X indicates mice already dead at the analysis. (C) Mean ± standard error of the mean for total flux (photons per second [p/s]) from transferred Foxp3Luc+ cells in indicated groups. Data pooled from 3 independent experiments. **P < .01 calculated by log-rank test between indicated 2 groups (A) or by 2-way analysis of variance between Ctrl-lipo and α-GalCer-lipo at indicated time point (C), ***P < .001 by 2-way analysis of variance between Ctrl-lipo and α-GalCer-lipo at indicated time point. BMC, BM cell; TBI, total-body irradiation.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/5/11/10.1182_bloodadvances.2020003272/3/m_advancesadv2020003272f3.png?Expires=1769156704&Signature=Z1x3bqX~UJvDy~D~iZHN3kK0s443AMs~MgI0MXCypFtnTnDlKGpdoXDxs~RzvjIDLENmIaNDnxGduDggvQk1LaZC8inYtdaRuhFmy96oGK77OBPl6bp36VS~anNAevrwyLwIIrxaZNEn8LVK8giZyFLoHVzqNkWmCYnqdyNlohjZC77dn84j-Imte1ZihEbixMqzvVrzZDFBgdiVOJOgh2~X0daf72WFIfOayGNaVhafXid~VDM8Be0ocjlUZvbW8LyigjE3CoS198e8yuCuZ7UQKBIvM3Un6H4u2y8kLfvF8CL1mz0VGLg7-lShJG1xRlmc8PsZJ4kZbiSL6RnPoA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Tregs accumulate in mLNs in α-GalCer-lipo–treated mice. Recipient mice received Foxp3GFP+Luc+ cells with Ctrl-lipo (blue; n = 7) or α-GalCer-lipo (red; n = 7) and were euthanized 5 minutes after luciferase injection on day 10 after BMT. Organs were removed and images were acquired within 15 minutes after luciferase injection. (A) Representative images of ex vivo BLI. Far right box indicates region of interest (ROI) for each organ. BM cell (BMC; gray; n = 6) control without Tcon/Foxp3GFP+Luc+ Treg transfer. (B) Mean ± standard deviation of total flux (photons per second [p/s]) obtained from each ROI. Data pooled from 2 independent experiments. (C) Images of immunofluorescent staining of mLN for CD4 (Alexa Fluor 594; red), Foxp3GFP (Alexa Fluor 488; green), and 4′,6-diamidino-2-phenylindole (DAPI; blue). Left shows low magnification (10×; white bar indicates 100 μm); right shows high magnification of inset region (40×; white bar indicates 20 μm). *P < .05, **P < .01, ***P < .001 between indicated 2 groups calculated by ordinary 1-way analysis of variance with Holm-Sidak correction.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/5/11/10.1182_bloodadvances.2020003272/3/m_advancesadv2020003272f4.png?Expires=1769156704&Signature=266iQDGlnr0Nu~86JVqdyfh2m8vmoDYTikUrLYaEZ5rJiXesMuz1bEGVR5tLiIYbvIpzNofp4krFnyUsjlaGyV1vdvWoCBYedRRaRFWckBNRBG5gTyPjtSHhbjBNLAkPer-xJfmUgiXkF7uo9pcceOiIRjw~nE~goHNF8sk83c1bscyZbUpc2uoC97HR~zXAxbkJUvqODhK~C9QLC80QddXCVlU5CmymAibXrdBHgs8j~OfTcGBmEa6~ZqiwN75VhV6q8WPnDlgRf1Q2oQGwoyi6L6O~NIbhUBzphmtYUGjdujhJUrZQYrp~PJa5i0ETameq9nUfQRIYXyqDP0NQjA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

