Key Points
We report the first case of coronavirus disease 2019 (COVID-19) in a multiple myeloma patient successfully treated with tocilizumab.
Although tocilizumab was effective in the treatment of COVID-19 in this case, randomized controlled trials are needed.
Introduction
Since December 2019, novel coronavirus pneumonia, named coronavirus disease 2019 (COVID-19) by the World Health Organization (WHO), has been a concerning global public health crisis. COVID-19 has become common in the elderly, frequently combining with chronic underlying disease.1 Because older patients with hematological malignancies are immunoincompetent, they are at high risk for novel coronavirus infection. Experimental research findings indicate that an excessive immune response and a strong cytokine storm, which may include high levels of granulocyte-macrophage colony-stimulating factor and interleukin-6 (IL-6), are activated in severe COVID-19.2 Here, we report the first case of COVID-19 in a patient with multiple myeloma (MM) successfully treated with the humanized anti–IL-6 receptor antibody tocilizumab.
Case description
A 60-year-old man working in Wuhan, China developed chest tightness without fever and cough on 1 February 2020. When he visited the local Wuhan hospital, he was admitted immediately after computed tomography (CT) imaging of his chest showed multiple ground-glass opacities and pneumatocele located in both subpleural spaces. He received 400 mg of moxifloxacin IV daily for 3 days. Nasopharyngeal swab specimens were collected to detect severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) nucleic acid. The swab specimens were tested by real-time reverse transcriptase–polymerase chain reaction; a positive result was received 3 days later. The patient was diagnosed with COVID-19, and was given 200-mg umifenovir (Arbidol) tablets orally, 3 times daily, for antiviral treatment.
The patient had a history of symptomatic MM (immunoglobulin Aλ [IgAλ], IgIIIA), which was diagnosed on 12 May 2015. At that time, a bone marrow aspirate showed 17.1% clonal plasma cells, and multiple osteolytic bone lesions obvious in frontal and temporal bone on radiography. His kidney biopsy confirmed amyloidosis; laboratory testing also showed proteinuria. The patient received 2 cycles of induction chemotherapy consisting of bortezomib, thalidomide, and dexamethasone, and his symptoms completely disappeared. After that, he refused bortezomib-based treatment and only received thalidomide for maintenance.
On 16 February 2020, the patient’s chest tightness was aggravated with shortness of breath as a result of decreased arterial oxygen saturation (∼93% at rest). He was immediately transferred to Unit Z6 in the cancer center of Wuhan Union Hospital. On admission to the cancer center, his physical examination results were as follows: body temperature, 36.6°C; pulse, 96 per minute; blood pressure, 145/95 mm Hg; and respiratory rate, 22 breaths per minute. Lung auscultation revealed lowered breath sound in the left lower lung. Laboratory tests showed lymphocytopenia (0.89 × 109/L); other parameters were approximately normal (Table 1). The patient’s illness was evaluated as severe.
Methods
For analysis, we collected the patient’s medical records, which included clinical characteristics, laboratory parameters, chest CT imaging, treatment approach, and clinical outcome. This case study was approved by the institutional review board of the First Affiliated Hospital of University of Science and Technology of China, and informed consent was obtained.
Results and discussion
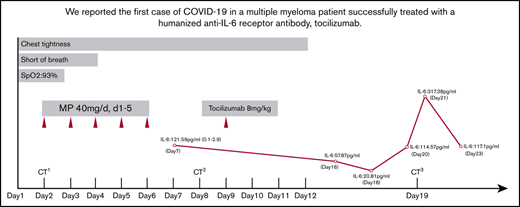
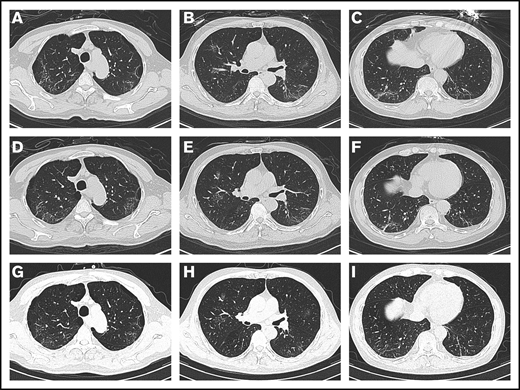
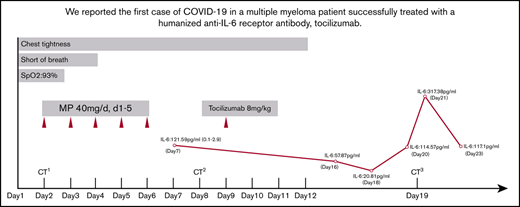
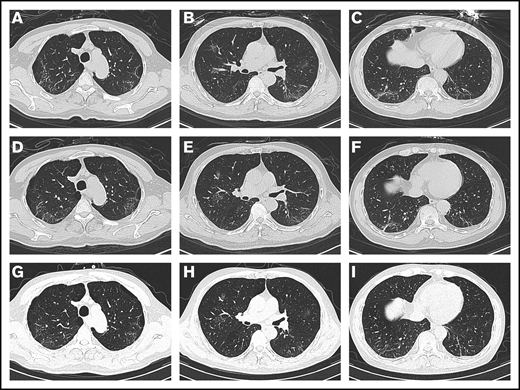
On admission, the patient’s arterial oxygen saturation increased to 96% with oxygen supplementation via nasal cannula (3 L/min). Chest CT imaging on hospital day 2 showed bilateral, multiple ground-glass opacities (Figure 1A-C). Considering his sustained chest tightness and shortness of breath, 40 mg of methylprednisolone, administered IV daily, was given on days 2 to 6. The patient then reported that his breathing had improved, but he still felt chest tightness. Chest CT imaging on hospital day 8 showed that the bilateral, multiple ground-glass opacities from the first scan remained (Figure 1D-F), and laboratory investigations revealed a high level of serum IL-6. On hospital day 9 (illness day 24), the patient was given 8 mg/kg tocilizumab, administered IV, 1 time. On hospital day 12, his chest tightness disappeared. After tocilizumab administration, the IL-6 level decreased gradually over the following 10 days (from 121.59 to 20.81 pg/mL), then increased rapidly to the peak (317.38 pg/mL), and then decreased to a low level (117.10 pg/mL) (Figure 2). The transient rebounding of the IL-6 level to the peak does not mean COVID-19 relapse: instead, this might be attributed to the recovery of the normal T cells. On hospital day 19, the patient had a third chest CT scan, which showed that the range of ground-glass opacities had obviously decreased (Figure 1G-I). The patient was declared to be cured and was discharged from the hospital on 13 March 2020. He had no symptoms of MM, and related laboratory findings were all in normal ranges (Table 1).
Chest CT images. (A-C) Chest CT imaging on hospital day 2 showed bilateral, multiple ground-glass opacities. (D-F) Chest CT imaging on hospital day 8 showed that the bilateral, multiple ground-glass opacities from the first scan were still there. (G-I) Chest CT imaging on hospital day 19 showed that the range of ground-glass opacities had obviously decreased after use of tocilizumab.
Chest CT images. (A-C) Chest CT imaging on hospital day 2 showed bilateral, multiple ground-glass opacities. (D-F) Chest CT imaging on hospital day 8 showed that the bilateral, multiple ground-glass opacities from the first scan were still there. (G-I) Chest CT imaging on hospital day 19 showed that the range of ground-glass opacities had obviously decreased after use of tocilizumab.
Timeline of symptoms, IL-6 level, and treatment after admission. CT1, first CT scan; CT2, second CT scan; CT3, third CT scan; MP, methylprednisolone; SpO2, peripheral oxygen saturation.
Timeline of symptoms, IL-6 level, and treatment after admission. CT1, first CT scan; CT2, second CT scan; CT3, third CT scan; MP, methylprednisolone; SpO2, peripheral oxygen saturation.
Patients with MM are at high risk for not only COVID-19 but also treatment difficulties. Common symptoms of COVID-19 include fever and cough,3,4 but the patient in our study had chest tightness and shortness of breath, indicating that the clinical symptoms of COVID-19 are not typical in patients with coexisting comorbidities such as MM. As one of the important cytokines that can lead to an acute severe systemic inflammatory reaction known as a cytokine storm, IL-6 can also active the coagulation pathway and inhibit myocardial function,5-7 much like the clinical characteristics of severe COVID-19. Zhou et al2 found that excessive immune responses by pathogenic T cells and inflammatory monocytes may associate with severe lung pathology, and speculated that a monoclonal antibody targeting the IL-6 receptor might be effective in the treatment of novel coronavirus pneumonia. Guan et al1 reported that mechanical ventilation was more common in patients with severe disease (32.4% noninvasive ventilation; 14.5% invasive ventilation).
Our patient’s clinical conditions gradually recovered after tocilizumab treatment, indicating that tocilizumab is effective in the treatment of novel coronavirus infection. IL-6 signaling plays a crucial role in the pathogenesis of MM, and small molecule inhibitors targeting IL-6 signaling are highly effective at preventing MM cell growth.8 We speculate that tocilizumab might also have potential benefit for MM as an immunotherapy in the future. This case is the first to prove that tocilizumab is effective in the treatment of COVID-19 in MM with obvious clinical recovery; however, randomized controlled trials are needed to determine the safety and efficacy of tocilizumab.
Data-sharing requests may be e-mailed to the corresponding author, Changcheng Zheng, at zhengchch1123@ustc.edu.cn.
Acknowledgment
The authors thank all of the medical staff members involved in treating this patient.
Authorship
Contribution: X.Z. and C.Z. designed and performed the research, analyzed and interpreted the data, and wrote the paper; and K.S., F.T., M.F., H.G., Z.L., and J.W. performed the research and analyzed and interpreted the data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Changcheng Zheng, Department of Hematology, The First Affiliated Hospital of University of Science and Technology of China, 17 Lujiang Rd, Hefei 230001, China; e-mail: zhengchch1123@ustc.edu.cn.
References
Author notes
X.Z. and K.S. contributed equally to this work.