Key Points
HCT survivors (≥2 years) have a significant, persistent burden of infections vs other cancer survivors and the general population.
Vaccine-preventable late infections were 3 times greater in HCT vs non-HCT survivors and >30 times greater than in the general population.
Abstract
Few studies have compared the incidence of infections occurring ≥2 years after hematopoietic cell transplant (HCT) with other cancer patients and the general population. In this study, ≥2-year HCT survivors who were Washington residents treated from 1992 through 2009 (n = 1792; median age, 46 years; 52% allogeneic; 90% hematologic malignancies) were matched to individuals from the state cancer registry (n = 5455, non-HCT) and driver’s license files (n = 16 340; Department of Licensing [DOL]). Based on hospital and death registry codes, incidence rate ratios (IRRs; 95% confidence interval [CI]) of infections by organism type and organ system were estimated using Poisson regression. With 7-year median follow-up, the incidence rate (per 1000 person-years) of all infections was 65.4 for HCT survivors vs 39.6 for the non-HCT group (IRR, 1.6; 95% CI, 1.3-1.9) and 7.2 for DOL (IRR, 10.0; 95% CI, 8.3-12.1). Bacterial and fungal infections were each 70% more common in HCT vs non-HCT cancer survivors (IRR, 1.7; P < .01), whereas the risk for viral infection was lower (IRR, 1.4; P = .07). Among potentially vaccine-preventable organisms, the IRR was 3.0 (95% CI, 2.1-4.3) vs the non-HCT group. Although the incidences of all infections decreased with time, the relative risk in almost all categories remained significantly increased in ≥5-year HCT survivors vs other groups. Risk factors for late infection included history of relapse and for some infections, history of chronic graft-versus-host disease. Providers caring for HCT survivors should maintain vigilance for infections and ensure adherence to antimicrobial prophylaxis and vaccination guidelines.
Introduction
Hematopoietic cell transplantation (HCT) can be a curative option for patients with high-risk cancers and other life-threatening disorders. Advances in transplantation have led to an increasing number of long-term survivors, estimated to increase to >500 000 by 2030.1 Nevertheless, late complications associated with high morbidity and mortality remain commonplace, and infections cause 7% to 13% of deaths among transplantation survivors within 3 years of HCT.2
Even after engraftment is achieved, complete immune reconstitution may take months if not years, particularly among allogeneic HCT recipients affected by chronic graft-versus-host disease (cGVHD) and who require prolonged immunosuppression.3 Delayed immune recovery has also been reported in autologous HCT recipients.3 However, most publications on late events after HCT have focused on relapse of original malignancy, cGVHD, and new cancers.4-7 Studies addressing the specific types of infections that occur years after HCT remain limited.3,8,9 We previously reported that a large single-center cohort of long-term (≥2-year) allogeneic and autologous HCT survivors experienced a significantly greater risk of infections compared with matched non-HCT cancer survivors and a matched general population sample.10 The analysis presented herein extends those earlier findings by examining in depth the types of infections experienced and the HCT-specific factors associated with this increased risk. This includes examining associations specific to allogeneic and autologous recipients. As the population of long-term HCT survivors grows, it becomes important to better describe these late complications, which may then lead to improved prevention strategies.11,12
Methods
HCT survivor and comparison cohorts
HCT survivors were Washington residents treated at the Fred Hutchinson Cancer Research Center (FHCRC), a National Cancer Institute–designated comprehensive cancer center and the only accredited institution that performs allogeneic HCT in Washington. Research was approved by the Washington State Institutional Review Board. A few other institutions perform autologous HCT only. The FHCRC HCT database was screened to identify patients who received allogeneic and autologous HCT from 1992 through 2009 (n = 7108), survived ≥2 years after transplantation (n = 4081), and were state residents at the time of transplantation (n = 1929) for a malignant condition (n = 1792). Race/ethnicity, pretransplantation diagnosis, transplant-related exposures (donor type, stem cell source, conditioning regimen, and cGVHD requiring systemic immunosuppressive treatment) were obtained from the FHCRC database.
A comparison cohort of patients with cancer was identified from the Washington State Cancer Registry (non-HCT group), a population-based cancer incidence registry affiliated with the National Program of Cancer Registries. It should be noted that cancer treatment duration and relapse status are not available from the state cancer registry. After excluding records of FHCRC HCT survivors, the remaining state residents with an initial cancer diagnosis from 1992 to 2009 and survived ≥2 years were randomly selected and frequency matched to HCT survivors at an ∼3:1 ratio by sex, age at cancer diagnosis/HCT (10-year age increments plus age <20 and ≥60), year of cancer diagnosis/HCT (2-year increments), and cancer diagnosis group (n = 5455; supplemental Table 1). A separate population-based noncancer comparison cohort was drawn from the Washington State Department of Licensing (DOL) files for 1992 to 2009, excluding persons within the HCT or non-HCT groups. Subjects were randomly selected from DOL files to be frequency matched (10:1 ratio; n = 16 340) on their age at and year of driver’s license issuance or renewal to HCT survivors who were state residents and ≥16 years of age at HCT by sex and age at and year of HCT (n = 1634). State death records were screened to ensure that the comparison subjects did not die within 2 years after licensing.
Outcomes ascertainment
To assess hospitalizations, we screened the Washington State Comprehensive Hospital Abstract Reporting System, which contains all hospital discharges from non-Federal facilities. This database contains up to 9 diagnosis codes (International Classification of Diseases, ninth revision [ICD-9]) for each hospitalization during the years of study. Identifiers allowed longitudinal tracking of individuals across multiple hospitalizations and linkage to outside data sources. To assess mortality among all subjects, we screened the state death registry, which records the primary and up to 6 contributing causes of death (ICD-9 and 10th revision) and includes out-of-state deaths of residents. Similar to our previous work,10 we used a sequential deterministic linkage strategy to link survivors and comparison subjects to hospital discharge and death records from 1992 through 2011. Variables used for linkage included components of names, birth date, sex, zip code, county of residence, date of HCT, corresponding hospitalization, and date of death (if applicable). We then assigned linked ICD-9 and equivalent ICD-10 codes to major infection categories (supplemental Table 2) occurring ≥2 years after the index date (ie, transplantation for the HCT group, cancer diagnosis for the non-HCT group, and driver’s licensing date for the DOL group). This included a category corresponding to potentially vaccine-preventable infections (ie, Bordetella pertussis, Haemophilus influenzae, hepatitis A and B viruses, human papilloma virus, influenza A and B viruses, measles virus, mumps virus, Neisseria meningitidis, rubella virus, Streptococcus pneumoniae, and Varicella zoster), acknowledging that the composition of some vaccines evolved over this time period and some were introduced only toward the end of the study period. Note that organisms in this vaccine-preventable category were also counted in other outcome categories (eg, V zoster was included in the general viral infections category; supplemental Table 2).
Statistical analysis
Follow-up of individuals without events was censored on 31 December 2011, or earlier if they died of any cause other than the specific outcome category examined.13 We reported the incidence rate of infections per 1000 person-years [PY] overall and by time since the index date (2-4, 5-9, and ≥10 years since HCT, cancer diagnosis, or driver’s license issuance or renewal). We used Poisson regression to estimate the incidence rate ratio (IRR) and associated 95% confidence interval (CI), comparing infection rates between cohorts.14 Models were adjusted for sex and year of and age at the index event, and for HCT vs non-HCT cancer survivors, the underlying diagnosis group.15 In analyses restricted to HCT recipients, we analyzed patients by allogeneic and autologous donor status separately and also examined the contributions of race/ethnicity, relapse of the original disease within the first 2 years after HCT, and presence of cGVHD (allogeneic survivors only). Subanalyses examined these relationships among ≥5-year survivors. We used STATA, version 15, for all analyses (StataCorp, College Station, TX).
Results
The age and sex of the survivor and DOL comparison groups were similar by design (Table 1). The median age at HCT was 46 years vs 49 years in the non-HCT group at cancer diagnosis and 48 years in the DOL group. Among surviving individuals, the HCT group had a median follow-up of 7.1 years vs 7.2 and 7.7 years for the non-HCT and DOL groups, respectively (range, 2-20 for all groups).
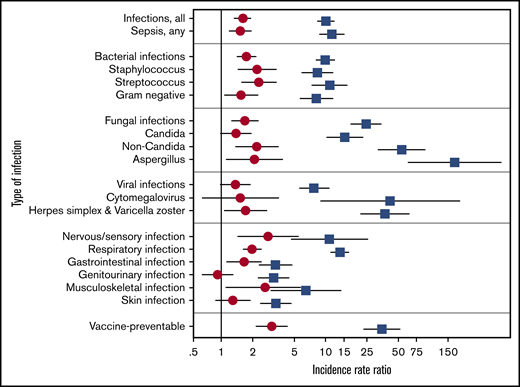
Based on >2500 unique infection events, the incidence rate (per 1000 PY) of all infections was 65.4 in HCT survivors vs 39.6 in the non-HCT group and 7.2 in the DOL group (Table 2). This corresponded to an IRR of 1.6 (95% CI, 1.3-1.9) for HCT vs non-HCT and 10.0 (95% CI, 8.3-12.1) for HCT vs DOL (Figure 1; supplemental Table 3). Among those with hematologic malignancies only, the IRR for HCT vs non-HCT was 1.8 (95% CI, 1.2-1.8), whereas if limited to solid tumors, the IRR was 4.6 (95% CI, 2.6-8.2). When pathogen-specific risks were analyzed, bacterial and fungal infections were each 70% more common in HCT vs non-HCT cancer survivors (IRR, 1.7; P < .01). These included infections attributed to Staphylococcus sp., Streptococcus sp., and non-Candida fungi, including Aspergillus sp., with IRRs ranging from 2.1 to 2.3 (P < .05). Differences in viral infection rates were more modest (IRR, 1.4; 95% CI, 0.98-1.9; P = .07).
Comparative IRRs of the 3 study groups. IRR with 95% CI of infections among ≥2-year HCT survivors vs non-HCT cancer survivors (circles), and HCT survivors vs the general population (squares). Estimates included adjustment for sex, age, and underlying cancer diagnosis and HCT/cancer diagnosis year (if applicable). IRRs with 95% CI that do not cross 1 are statistically significant.
Comparative IRRs of the 3 study groups. IRR with 95% CI of infections among ≥2-year HCT survivors vs non-HCT cancer survivors (circles), and HCT survivors vs the general population (squares). Estimates included adjustment for sex, age, and underlying cancer diagnosis and HCT/cancer diagnosis year (if applicable). IRRs with 95% CI that do not cross 1 are statistically significant.
When categorized by organ systems, respiratory infections were significantly more common in the HCT vs the non-HCT group (Table 2), with an IRR of 2.0 (95% CI, 1.6-2.4; Figure 1; supplemental Table 3). This included all pneumonia subtypes whether classified as bacterial, fungal, or viral, including a higher incidence rate (per 1000 PYs) of Pneumocystis jiroveci infection: HCT 1.5 (95% CI, 0.9-2.5) vs non-HCT 0.3 (95% CI, 0.2-0.6) and DOL 0.03 (95% CI, 0.01-0.08). IRRs for infections by other organ systems also showed increased incidences by 70% to 180% in the HCT group compared with the non-HCT group, including those involving the nervous/sensory (IRR, 2.8; 95% CI, 1.4-5.5), gastrointestinal (IRR, 1.7; 95% CI, 1.1-2.4), and musculoskeletal (IRR, 2.6; 95% CI, 1.1-6.3) systems. For all categories assessed by organ system (and by organism), IRRs were substantially greater in HCT survivors than in the DOL group, with many exceeding 10.
When potentially vaccine-preventable infections were examined, HCT survivors had increased rates of H influenza, S pneumoniae, viral influenza, and V zoster (Table 3), with associated IRRs of 3.0 (95% CI, 2.1-4.3) and 34.6 (95% CI, 23.0-52.2), compared with the non-HCT and the DOL groups, respectively. Even if hepatitis B and V zoster were excluded, these IRRs remained significant with associated IRRs of 4.4 (95% CI, 2.8-7.0) and 28.2 (95% CI, 16.9-47.1), compared with the non-HCT and the DOL groups, respectively.
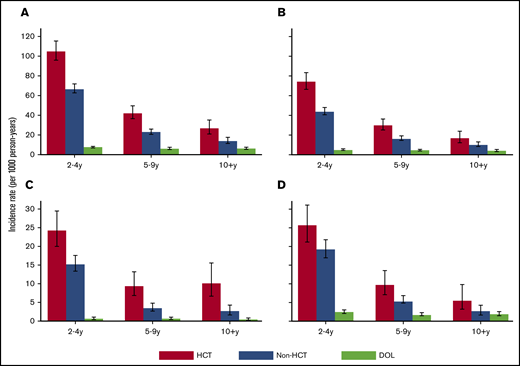
When the incidence of infections was examined over time, rates for both HCT and non-HCT cancer survivors decreased over time, although the absolute rates generally remained greater for HCT survivors vs non-HCT survivors and the DOL group, even after 10 years (Figures 2 and 3). When data were limited to ≥5-year survivors in both the HCT and non-HCT group, the IRRs associated with HCT vs non-HCT cancer survivors remained increased, suggesting that the relative risks in HCT survivors did not diminish with increased time since HCT (supplemental Table 3).
Rates of infection by organism type. Incidence rate with 95% CI of infections by organism type among HCT survivors, non-HCT cancer survivors, and the general population (DOL), shown separately by time since index date: all infections (A), bacterial (B), fungal (C), and viral (D). The y-axis ranges vary among the panels.
Rates of infection by organism type. Incidence rate with 95% CI of infections by organism type among HCT survivors, non-HCT cancer survivors, and the general population (DOL), shown separately by time since index date: all infections (A), bacterial (B), fungal (C), and viral (D). The y-axis ranges vary among the panels.
Rates of infection by organ type. Incidence rate of infections with 95% CI by organ system among hematopoietic cell transplantation (HCT) survivors, non-HCT cancer survivors, and the general population (DOL), shown separately by time since index date: respiratory (A), genitourinary (B), gastrointestinal (C), skin (D), nervous/sensory (E), and musculoskeletal (F). The y-axis range may vary among the panels.
Rates of infection by organ type. Incidence rate of infections with 95% CI by organ system among hematopoietic cell transplantation (HCT) survivors, non-HCT cancer survivors, and the general population (DOL), shown separately by time since index date: respiratory (A), genitourinary (B), gastrointestinal (C), skin (D), nervous/sensory (E), and musculoskeletal (F). The y-axis range may vary among the panels.
When we examined HCT-specific risk factors for infection among ≥2-year allogeneic HCT survivors, a history of cGVHD was not independently associated with an overall increased risk of infection (IRR, 1.6; 95% CI, 0.9-2.8; Table 4). However, cGVHD was associated with sepsis (IRR, 1.9; 95% CI, 1.0-3.8), viral infection (IRR, 2.4; 95% CI, 1.1-5.3), bacterial pneumonia (IRR, 2.2; 95% CI, 1.0-4.9), genitourinary infection (IRR, 2.7; 95% CI, 1.0-6.8), and vaccine-preventable infection (IRR, 2.9; 95% CI, 1.3-6.6). When our analysis was restricted to ≥5-year allogeneic HCT survivors, the risk of infection associated with a history of cGVHD was more attenuated and no longer statistically significant for any outcome, although IRRs for vaccine-preventable infections remained ∼3. Relapse of the original cancer was associated with an increased risk of almost all infection categories for both allogeneic and autologous ≥2-year HCT survivors. However, although history of relapse remained a significant infection risk factor in the analysis limited to ≥5-year autologous survivors, relapse was no longer significant for ≥5-year allogeneic survivors. Even if HCT survivors with a history of relapse are excluded, the HCT group continued to have an increased risk of infections vs non-HCT cancer survivors (for whom relapse data are unavailable): ≥2-year survivors, IRR 1.3 (95% CI, 1.0-1.6), and ≥5-year survivors, IRR 1.8 (95% CI, 1.3-2.5).
Discussion
Prior studies have suggested that the incidence of infections occurring the first year after HCT may be as low as 5% to 8% and may affect only certain high-risk groups.9,11,16,17 However, our prior work showed that long-term HCT survivors had a 10-year cumulative incidence of infectious complications >30% (vs 9% in non-HCT cancer survivors).10 In this follow-up analysis, we found that HCT survivors had a significantly increased risk of bacterial, fungal, and viral infections compared with matched non-HCT cancer survivors. When analyzed by organ system, we showed that infections of almost every organ system were also more common among HCT survivors compared with non-HCT cancer survivors. This risk persisted, even when restricted to ≥5-year survivors. Notably, the relative risks for vaccine-preventable infection in the HCT group remained significantly increased with longer follow-up, suggesting a potential area for intervention, such as improving adherence and education regarding primary and booster vaccinations. To our knowledge, this is the most comprehensive description and comparison of infectious complications occurring in long-term HCT survivors vs non-HCT cancer and noncancer populations in the literature.
The importance of late infectious complications after transplantation has evolved over time, as long-term survival has become the norm for most HCT recipients. For infections occurring >2 years post-HCT, Robin et al9 performed a retrospective review of 196 matched-related transplant recipients, and found that the 8-year incidence of bacterial infections was 15%; for viral infections, 35%; and for fungal infections, nearly 4%. Among the infection subtypes analyzed, hepatitis C virus and V zoster were the most common documented viral infections and Aspergillus and Candida were the most common documented fungal infections. Most bacterial infections were specified as “undocumented.” Using the Center for International Blood and Marrow Transplant Research data set, Norkin et al8 analyzed the outcomes of over 15 000 ≥2-year HCT survivors and found that infections were the primary or a contributory cause of death ∼30% of the time. Among the 564 deaths attributed to infection, bacterial causes were the most common (nearly 40%) followed by viral and fungal causes (∼10% each).
A major limitation of most studies of late post-HCT infectious complications is the lack of a comparison group. An analysis using a data set of community adults in the United States compared sepsis in ∼3000 cancer survivors (self-reported history of cancer off treatment of >2 years) with those without cancer history living in the same community.18 The risk of sepsis was 3 times greater in cancer survivors than in those without a history of cancer. However, this study did not include detailed information on cancer history. The Childhood Cancer Survivor Study (n > 12 000) found that nearly one-third of survivors reported infectious complications >5 years after cancer diagnosis, mostly sinopulmonary infection, followed by gastrointestinal infection.19 Compared with siblings, these childhood cancer survivors had a 30% increased risk of having late infection, including a threefold increased risk of death due to infection. Total-body irradiation (as a surrogate marker for HCT) was the greatest risk factor for late infection-related death in this study. Overall, our results are consistent with these findings.
Abnormalities or delays in immune reconstitution most likely play major roles in infection-related complications that occur after HCT. Despite normalization of the white blood cell count soon after successful engraftment, fully functional immunity after HCT may take many months if not years to recover.3 Multiple factors including patient age, underlying disease, history of infections, type of conditioning regimen, donor choice (especially cord blood), HLA-matching, graft processing (eg, T-cell depletion), immunosuppression, and infection prophylaxis guidelines can alter immune recovery.3 In most post-HCT patients, immune reconstitution occurs within the first 1 to 2 years, but some patients still have laboratory-detectable immune defects, even beyond 10 years.3 cGVHD and its treatment are major risk factors for poor immune reconstitution.11,20 In our allogeneic HCT cohort, cGVHD history was generally associated with increased infection risk, even if not all estimates were statistically significant.
Our data showed that there was an increased incidence of potentially vaccine-preventable infection. Because some of these diseases are treated in the outpatient setting, our methodology may have underestimated the infectious burden for HCT survivors. Antibody titers to vaccine-preventable diseases have been noted to be reduced or even undetectable after both allogeneic and autologous HCT and have been shown to decline further unless survivors are revaccinated.21 As a result, multiple organizations have established consensus recommendations on the revaccination of HCT survivors.22-25 Limited data are available to guide the timing of post-HCT vaccinations, but in general, vaccines containing live virus are contraindicated for both autologous and allogeneic HCT survivors within 24 months of transplant, or at any point for individuals with active GVHD and/or on systemic immunosuppression. However, those with GVHD should continue to be revaccinated with inactivated vaccine, even if vaccination start times are delayed relative to other HCT survivors because of delayed or incomplete immune reconstitution. Postvaccination assessment of titers, both in the midst of revaccination and in the long-term, has been recommended, although what to do for those patients who lack robust responses is less clear.21,23,26 Although our study lacked information on vaccination status and titers, future research should attempt to determine whether immune titers can be used to identify cancer survivors who may have an increased risk of potentially preventable infections.
Our results should be interpreted with several other limitations in mind. Newer regimens introduced after 2009, particularly reduced intensity conditioning that have become common, may be associated with reduced infection risk. Our study also would not have ascertained less serious infections occurring solely in the ambulatory setting. At the same time, it is possible that HCT and non-HCT cancer survivors may be more likely to be hospitalized as part of their diagnostic workup, compared with the general population, which may lead to an overestimate of the incidence of some conditions. However, this consideration should not greatly affect estimates for more serious infectious outcomes (eg, sepsis, Aspergillus). We also lacked reliable information on when survivors with cGVHD discontinued systemic immunosuppression. However, the effect of any differential misclassification of immunosuppressive status on late infection risk is difficult to know for certain. Nevertheless, we still found that infection risk in HCT survivors vs non-HCT cancer survivors remained increased 5 years after HCT, when the burden of cGVHD requiring systemic immunosuppression is reduced.27,28
A major strength of this study was its reliance on prospectively coded and population-based data sources free of response biases. In studies that rely on patients or primary care providers to provide long-term follow-up information, the accuracy of infectious outcomes and the ability to capture detailed infection data in ambulatory encounters has generally been noted to decrease 100 days after HCT when patients often leave the transplant center and return to their primary physicians.3,29 Administrative codes for infection are generally accurate, particularly when multiple diagnostic codes are applied to generate broader categories.30-35
As overall outcomes and survival after HCT improve, late infections remain among the most important causes of late morbidity and mortality.36 Providers caring for long-term HCT survivors should maintain high vigilance for infections in this population and ensure adherence to existing HCT antimicrobial prophylaxis and vaccination guidelines. Long-term HCT survivors may benefit from more comprehensive assessments of immune function to more clearly identify those individuals who remain at risk for these late infections.
Results were presented in part at the 55th annual meeting of the American Society of Clinical Oncology, Chicago, IL, 31 May to 6 June 2019.
Original data are available by e-mail request to the corresponding author.
Acknowledgments
This work was supported by National Institutes of Health (NIH), National Cancer Institute, grants CA151775, CA167451, and CA18029. A large portion of the data provided by the Washington State Cancer Registry were collected under NIH Surveillance, Epidemiology, and End Results (SEER) program contracts, National Cancer Institute grants N01-CN-67009 and N01-PC-35142, and Department of Health and Human Services/NIH grant HHSN26120130012I.
Authorship
Contributions: A.M.F., B.A.M., J.A.H., and E.J.C. designed the research; M.J.B., P.A.C., M.E.D.F., S.J.L., and E.J.C. provided the data; K.L.C.-H., W.M.L., and E.J.C. performed the primary analyses; and all authors reviewed the data analyses and wrote the paper.
Conflict-of-interest disclosure: The following authors report disclosures not directly relevant to this publication: M.J.B. (Helocyte, Pulmocide, Chimerix, Gilead, Janssen, ReViral, Allovir, ADMA, Symbio, VirBio, Moderna, Ansun Biopharma, Merck, Astellas, GlaxoSmithKline, Takeda), P.A.C. (Incyte, Mallinckrodt, Mesoblast, Pharmacyclics), M.E.D.F. (Astellas, Mallinckrodt, CSL Behring, Pharmacyclics, Incyte, Therakos),S.J.L. (Mallinckrodt, EMD Serono, Genzyme, Incyte, Kadmon, MSD Oncology, Pfizer, Regeneron, Sanofi, Amgen, Bristol-Myers Squibb, Takeda), and B.A.M. (AstraZeneca). The remaining authors declare no competing financial interests.
Correspondence: Eric J. Chow, Fred Hutchinson Cancer Research Center, PO Box 19024, M4-C308, Seattle, WA 98109; e-mail: ericchow@uw.edu.
References
Author notes
The full-text version of this article contains a data supplement.