TO THE EDITOR:
During allogeneic hematopoietic cell transplantation (HCT), the diversity of the intestinal flora is disrupted, largely because of antibiotic administration.1,2 Antibiotics with anaerobic coverage have a more profound effect on intestinal microbiota.1-3 Loss of intestinal microbiome diversity contributes to the risk of subsequent infections, graft-versus-host disease (GVHD), and increased mortality following HCT.1-9
In a recent issue of Blood Advances, Zhang et al reported a novel association between vancomycin use and risk of cytomegalovirus (CMV) reactivation among CMV-seropositive HCT recipients.10 The authors proposed that some Gram-positive bacteria might protect against CMV reactivation. However, the use of fluoroquinolones, cephalosporins, piperacillin-tazobactam, carbapenems, or clindamycin, all of which have a broad spectrum of activity against Gram-positive bacteria, was not associated with risk of CMV. In addition, the authors did not perform analyses assessing risk of clinically significant CMV infection (cs-CMVi; defined as CMV disease11 or CMV viremia leading to preemptive treatment), the preferred primary end point in CMV clinical trials12 and arguably a more relevant outcome. This is important because >25% of patients with CMV viremia experience spontaneous clearance (ie, without need for therapy).13,14 Although CMV reactivation (any viremia) is an important outcome because even low levels of CMV DNA have been associated with increased mortality,15 the impact of antibiotic use on risk of cs-CMVi remains to be studied.
We reviewed antibacterial antibiotic use in the first 14 days (day 0 to +14) posttransplant in a previously described cohort of 174 allogeneic HCT recipients with available information on rates of CMV reactivation.13 After excluding 1 patient who died within 14 days of transplant and restricting analyses to CMV seropositive (R+) patients, a total of 149 subjects were included (supplemental Table 1). Similar to previous reports,16 the rate of CMV was nearly identical among CMV D+/R+ and D−/R+ groups (supplemental Figure 1), and all episodes of CMV reactivation (n = 107) occurred before day +100 (median, 17 days; interquartile range, 7-30 days); cs-CMVi was higher among D−/R+, but this finding was not statistically significant (supplemental Figure 1). Because median time to engraftment was 11 days (interquartile range, 10-14), using antibiotic exposure by day +14 as predictor allowed us to capture most of the antibiotic exposures in the setting of prophylaxis or neutropenic fever episodes, which typically occur in the preengraftment period. Antibiotic prophylaxis consisted of levofloxacin 500 mg daily from day −3 until neutrophil engraftment or initiation of neutropenic fever treatment with cefepime. Protocol modifications such as escalation of therapy occurred at the discretion of the treating physician. All the patients received antiviral prophylaxis with acyclovir 800 mg orally twice daily; none of the patients received anti-CMV prophylaxis. CMV monitoring and preemptive therapy were performed per local protocol.13 The study was conducted consistent with Declaration of Helsinki principles. Kaplan-Meier plots with a log-rank test, Mann-Whitney U, and Fisher’s exact tests were performed using GraphPad Prism Software, version 7.03. Cox proportional hazards regression and logistic regression analyses were performed using SAS, version 9.4.
All the patients received at least 1 type of antibacterial antibiotic during the first 14 days posttransplant. Similar to previous reports,17 the most common antibiotics administered were levofloxacin (94%), intravenous vancomycin (60%), cefepime (53%), meropenem (16%), metronidazole (15%), clindamycin (5%), and piperacillin-tazobactam (3%) (supplemental Figure 2). Fifty-six (38%) patients received antibiotics as prophylaxis only; others underwent antibiotic escalation for culture-negative neutropenic fever in 67 (45%), bacteremia in 18 (12%), and other reason in 8 (5%) cases. Univariate analyses for single-antibiotic exposures are shown in supplemental Figures 3-6.
We were unable to establish an association between intravenous vancomycin use and CMV (any viremia) within 100 days post-HCT (supplemental Figure 3C; Table 1), even when restricting analysis to D−R+ patients (58% vs 71% for vancomycin [n = 30] vs no vancomycin [n = 14], respectively; P = .79). Vancomycin use was associated with cs-CMVi in univariate (supplemental Figure 4C) but not multivariate analyses (Table 1). Discordant findings with those by Zhang et al10 might have occurred for several reasons: their cohort was enriched for umbilical cord recipients; we used the contemporary IU/mL instead of copies/mL as CMV reporting units, and possibly different polymerase chain reaction platforms; many patients received anti-CMV prophylaxis in Zhang et al,10 whereas we used exclusively a preemptive approach; patients in our cohort were transplanted within a 4-year period (vs 8-year period in Zhang et al) and therefore less likely to be influenced by variations in clinical practice over time. Finally, inpatient CMV surveillance at our institution was conducted twice a week, which might have allowed us to capture more CMV events.
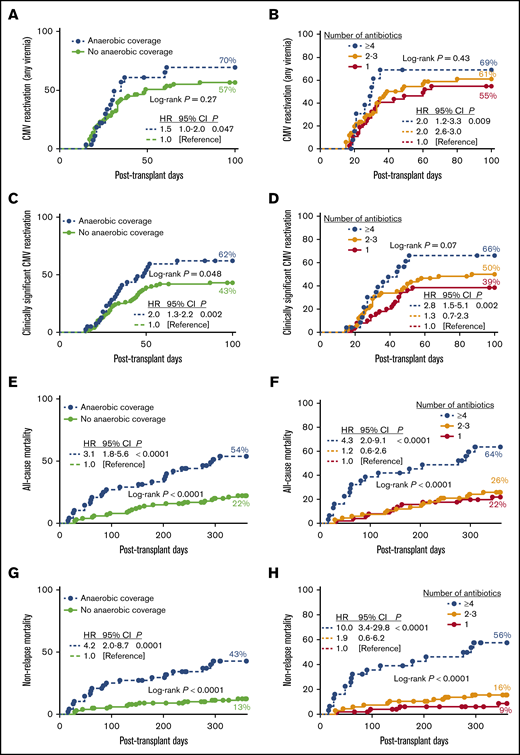
Administration of antibiotics with anaerobic coverage (ie, metronidazole, clindamycin, meropenem, and piperacillin-tazobactam) was associated with an increased risk of CMV (any viremia; Figure 1A), but this observation was not significant in the multivariate analysis (Table 1). Notably, administration of anaerobic antibiotics in the first 14 days posttransplant was associated with a twofold increase in the risk of cs-CMVi (Figure 1C), even after adjusting for known risk factors for CMV such as mismatched/unrelated donor, CMV seronegative donor, lymphoid malignancy, and GVHD (Table 1).
Early antibiotic exposure and clinical outcomes following HCT. (A-B) The 100-day cumulative incidence of CMV reactivation (any viremia) by antibiotic use by day +14. Number of subjects in log-rank analysis was as follows: anaerobic coverage (n = 28); no anaerobic coverage (n = 74); and >4 (n = 14), 2-3 (n = 51), or 1 (n = 37) antibiotic. (C-D) The 100-day cumulative incidence of clinically significant CMV reactivation by antibiotic use by day +14. Number of subjects in log-rank analysis was as follows: anaerobic coverage (n = 40); no anaerobic coverage (n = 100); and >4 (n = 24), 2-3 (n = 66), or 1 (n = 50) antibiotic. (E-F) One-year cumulative incidence of all-cause mortality by antibiotic use by day +14. Number of subjects in log-rank analysis was as follows: anaerobic coverage (n = 48); no anaerobic coverage (n = 101); >4 (n = 31), 2-3 (n = 67), or 1 (n = 51) antibiotics. (G-H) One-year cumulative incidence of nonrelapse mortality by antibiotic use by day +14. Number of subjects in log-rank analysis was as follows: anaerobic coverage (n = 48); no anaerobic coverage (n = 101); >4 (n = 31), 2-3 (n = 67) or 1 (n = 51) antibiotics. Insert corresponds to hazard ratios estimated by using Cox regression. Anaerobic coverage exposure includes metronidazole, clindamycin, meropenem, and piperacillin-tazobactam.
Early antibiotic exposure and clinical outcomes following HCT. (A-B) The 100-day cumulative incidence of CMV reactivation (any viremia) by antibiotic use by day +14. Number of subjects in log-rank analysis was as follows: anaerobic coverage (n = 28); no anaerobic coverage (n = 74); and >4 (n = 14), 2-3 (n = 51), or 1 (n = 37) antibiotic. (C-D) The 100-day cumulative incidence of clinically significant CMV reactivation by antibiotic use by day +14. Number of subjects in log-rank analysis was as follows: anaerobic coverage (n = 40); no anaerobic coverage (n = 100); and >4 (n = 24), 2-3 (n = 66), or 1 (n = 50) antibiotic. (E-F) One-year cumulative incidence of all-cause mortality by antibiotic use by day +14. Number of subjects in log-rank analysis was as follows: anaerobic coverage (n = 48); no anaerobic coverage (n = 101); >4 (n = 31), 2-3 (n = 67), or 1 (n = 51) antibiotics. (G-H) One-year cumulative incidence of nonrelapse mortality by antibiotic use by day +14. Number of subjects in log-rank analysis was as follows: anaerobic coverage (n = 48); no anaerobic coverage (n = 101); >4 (n = 31), 2-3 (n = 67) or 1 (n = 51) antibiotics. Insert corresponds to hazard ratios estimated by using Cox regression. Anaerobic coverage exposure includes metronidazole, clindamycin, meropenem, and piperacillin-tazobactam.
One-year all-cause mortality was 32% and higher for those patients who received anaerobic antibiotics (Figure 1E; supplemental Figure 5). Exposure to anaerobic antibiotics during the first 14 days post-HCT was independently associated with a threefold increase in all-cause mortality (Table 1). One-year nonrelapse mortality (NRM) was 22%, and higher for patients who received meropenem, vancomycin, cefepime, metronidazole, piperacillin-tazobactam, or anaerobic antibiotics by day +14 (Figure 1G; supplemental Figure 6). Increased risk of NRM following exposure to anaerobic antibiotics remained significant after adjusting for age, type of donor, comorbidity score, and other variables (adjusted hazard ratio, 4.21; 95% confidence interval, 1.8-9.9; P = .0008; Table 1).
Grade 3-4 GVHD was more commonly observed with early administration of metronidazole (17% vs 6%, P = .09), vancomycin (12% vs 2%; P = .03), or meropenem (21% vs 6%, P = .03). Consistent with previous reports,2 occurrence of grade 3-4 GVHD was more common among patients who received anaerobic antibiotics by day +14 compared with those who did not (19% vs 3%; P = .002). In multivariate analysis, exposure to anaerobic antibiotics was associated with fivefold increase in risk of severe GVHD (Table 1).
The association between early antibiotic exposure and outcomes was dose dependent. The 100-day incidence of CMV reactivation (any viremia) and cs-CMVi were highest among patients who received ≥4 and lowest in patients who had single antibiotic exposure by day +14 (Figure 1B,D). Incidence of grade 3-4 GVHD was 0%, 10%, and 16% for patients who received 1, 2-3, and ≥4 antibiotics (P = .008) and 3%, 18%, and 20% for patients who received 0, 1, or ≥2 anaerobic antibiotics (P = .003) by day +14, respectively. Similarly, there was a stepwise increase in 1-year all-cause mortality and NRM in patients who were exposed to an escalating number of antibiotics, with a 10-fold increase in probability of NRM in those exposed to ≥4 antibiotics (Figure 1F,H). All-cause and NRM were 22%, 47%, and 80%, and 13%, 32%, and 80% for patients who received 0, 1, or >2 anaerobic antibiotics, respectively (P < .0001; supplemental Figure 7).
Although randomized trials comparing different antibiotic stewardship (eg, anaerobic-sparing) strategies are needed, these observations reinforce the notion that early administration of antibiotics, especially those with anaerobic coverage, negatively affect clinical outcomes in HCT recipients, and highlight the importance of rational antibiotic use in this vulnerable patient population.
Send data sharing requests via e-mail to the corresponding author, Jose F. Camargo (jfc31@med.miami.edu).
Acknowledgments:
The authors are indebted to all the patients who participated in the study and thank Humberto Elejalde for assistance with data collection.
This research was supported by a grant from the National Institutes of Health, National Cancer Institute (P30CA240139), the Kalish Family Foundation (K.V.K.), and Applebaum Foundation (J.F.C. and K.V.K.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contribution: J.F.C. and A.D.A. conceived and designed the study; J.F.C., A.D.A., Y.N., A.N., M.I.M., D.P., and K.V.K. acquired and analyzed the data; J.F.C., Y.N., and A.N. conducted statistical analyses; J.F.C. prepared the first draft of the manuscript; and all authors were involved in the revision of the draft manuscript and have agreed to the final content.
Conflict-of-interest disclosure: K.V.K. has served as an ad hoc consultant to Kite/Gilead, Novartis, Celgene, Atara, Kiadis, Kadmon, Incyte, Gamida Cell, and Takeda. M.I.M. is a consultant in the medical advisory board of Viracor Eurofins and has received research funding from Merck. The remaining authors declare no competing financial interests.
Correspondence: Jose F. Camargo, Division of Infectious Diseases, University of Miami Miller School of Medicine, 1120 NW 14th St, Miami, FL 33136; e-mail: jfc31@med.miami.edu.
References
Author notes
The full-text version of this article contains a data supplement.