Key Points
Transplantation-related mortality has fallen in the past 40 years, with the most substantial improvement occurring at about the year 2000.
In the matched-pairs analysis, nonrelapse mortality at 1 year was 24.4% in the 1990s and 9.5% from 2013 through 2016.
Abstract
We performed a study to find out how advances in modern medicine have improved the mortality risk of allogeneic stem cell transplantation. We analyzed major transplantation outcome parameters in adult patients on the European Society for Blood and Marrow Transplantation (EBMT) registry who had hematologic malignancies and had received transplants from matched sibling donors. We performed multivariate analyses using the Cox proportional-hazards model including known risk factors for nonrelapse mortality and a matched-pairs analysis. We identified 38 800 patients who fulfilled the inclusion criteria. Considerable changes in patient characteristics have occurred in the past decades, such as older age, different underlying diseases, and a higher proportion of patients with advanced disease. Major reasons for transplantation-related death in the 1980s were infectious complications and graft-versus-host disease. Nonrelapse mortality, measured at 1 year after transplantation, has decreased over time: 29.7% from 1980 through 1989, 24.4% from 1990 through 1999, 14.8% from 2000 through 2009, and 12.2% from 2010 through 2016. On multivariate analysis, the year of transplantation was associated with reduced nonrelapse mortality (P < .0001; hazard ratio [HR] [95% confidence interval (CI)], 0.8 [0.79-0.82], for 5-year intervals) and decreased overall mortality (P < .0001; HR [95% CI], 0.87 [0.86-0.88]. In the matched-pairs analysis of 3718 patients in each group, nonrelapse mortality at 1 year was 24.4% in the 1990s and 9.5% from 2013 through 2016 (P < .0001; HR [95% CI], 0.39 [0.34-0.43]). Transplantation-related mortality has decreased significantly in the past 40 years. These favorable data facilitate evidence-based treatment decisions on transplantation indications in the context of the availability of novel immunotherapies.
Introduction
Allogeneic stem cell transplantation (allo-SCT) is a standard procedure for the treatment of hematologic diseases and other illnesses. The use of allo-SCT is constantly increasing, with nearly 20 000 transplantations reported to the European Society for Blood and Marrow Transplantation (EBMT) per year.1 Allo-SCT has cured many patients with life-threatening diseases since its broader use in clinical medicine began ∼40 years ago. However, allo-SCT has a downside of causing considerable treatment-related mortality, mainly driven by graft-versus-host disease (GVHD), infectious complications, and conditioning-related toxicity. Therefore, the decision to choose allo-SCT can be difficult, and reliable information on the current nonrelapse mortality (NRM) risk is needed. Over the past few decades, considerable progress has been made in the management of GVHD, as well as in prevention and treatment of infectious complications in allo-SCT recipients.2,3 We performed a study using the largest real-life allo-SCT registry, to find out if the NRM risk has been reduced in modern times.
Methods
Study design and data collection
This is a retrospective multicenter analysis derived from the data set of the EBMT registry. The EBMT is a voluntary working group of more than 600 transplantation centers that are required to report regular follow-up on all consecutive stem cell transplantations. Audits are routinely performed to determine the accuracy of the data. The study was planned and approved by the Transplant Complications Working Party of the EBMT. All patients gave their written informed consent to use their personal information for research purposes. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Eligibility criteria for this analysis included patients older than 18 years of age at allo-SCT who had acute lymphoblastic leukemia, acute myeloid leukemia, lymphoma, myelodysplastic syndrome, or myeloproliferative neoplasms and underwent a first allo-SCT (previous autologous transplantation[s] allowed) with a transplant from a matched sibling donor obtained from either bone marrow or peripheral blood, from 1980 through 2016. The decision to exclusively include allo-SCT recipients from matched family donors was driven by the difficulty in assessing the influence of HLA disparity over time. There have been major improvements in HLA typing during the study period, making it impossible to match recipients of transplants from unrelated donors in different historic cohorts.
Exclusion criteria were lack of information on disease progression (status or date), conditioning (intensity or use of total body irradiation), status at transplantation, and in vivo T-cell depletion. Data collected included recipient and donor characteristics (age, sex, cytomegalovirus serostatus and Karnofsky performance status score), diagnosis and status at transplantation, interval from diagnosis to transplantation, and transplantation-related factors, including conditioning regimen, use of antithymocyte globulin or alemtuzumab before transplantation for in vivo T-cell depletion, stem cell source, ex vivo T-cell depletion, and posttransplantation GVHD prophylaxis. Grading of acute GVHD was performed according to established criteria.4 Chronic GVHD was classified as limited or extensive, according to published criteria.5 For the purpose of this study, all necessary data were collected according to the EBMT guidelines, using the EBMT Minimum Essential Data forms.
Statistical analysis
Study end points were NRM, overall survival, progression-free survival, relapse incidence, and incidence and severity of acute GVHD and chronic GVHD. The start time for all end points was the date of transplantation. NRM was defined as death without relapse/progression, and progression-free survival was defined as survival without relapse or progression. Probabilities of overall survival and progression-free survival were calculated by the Kaplan-Meier method. Cumulative incidence functions were used to estimate NRM and relapse incidence in a competing risk setting, with death and relapse competing with each other.6 For the estimation of the cumulative incidence of acute and chronic GVHD, relapse and death were considered to be competing events. Follow-up was truncated at 4 years for all patients, to avoid any bias related to a longer follow-up for patients who underwent transplantation in the early decades. Multivariate analyses were performed by using the Cox proportional hazards model for all end points. All factors known to be potential risk factors for NRM were included in the final models: previous autologous transplantation(s), stem cell source, diagnosis, complete remission at transplant, patient age, patient sex, donor sex, intensity of conditioning, total body irradiation, and in vivo T-cell depletion. Some factors that were not readily available, especially in old transplantation records, were not included: ex vivo T-cell depletion, cytomegalovirus status, and Karnofsky performance status score. In the matched-pair subgroup analysis comparing transplant recipients in the 1990s with those from 2013 through 2016, patients were matched 1:1 for diagnosis, status at transplant, sex, and age (5-year categories). All tests were 2-sided. Statistical analyses were performed with R 3.4.2 software (R Development Core Team, Vienna, Austria) packages.
Results
Patient characteristics and causes of death
We analyzed NRM over time in adult patients undergoing a first allo-SCT from a matched sibling donor for hematologic malignancy reported to the EBMT. We identified 38 800 patients who fulfilled the inclusion criteria. The quantitative assessment of time of transplantation and its association with outcome was made by assuming year of allo-SCT as a continuous linear variable. However, for better visualization in figures and for presentation in some of the tables, we built 4 historic patient cohorts: 1980 through 1989, 1990 through 1999, 2000 through 2009, and 2010 through 2016. Patient characteristics of the cohorts changed fundamentally over time (Table 1). More recent cohorts were characterized by a higher proportion of (1) older patients; (2) peripheral blood as the donor stem cell source; (3) lymphoma, myelodysplastic syndrome, or myeloproliferative diseases as underlying malignancies; (4) reduced-intensity conditioning; (5) chemotherapy-only conditioning (without total body irradiation); and (6) in vivo T-cell depletion with antithymocyte globulin or alemtuzumab. The causes of mortality are given in Table 2. The most frequent cause of death across all cohorts was relapse of the underlying malignancy, closely followed by infections and GVHD. As expected, a higher number of secondary malignancies was reported as the cause of death in patients in early transplantation cohorts, considering the longer observation period. However, in all cohorts, a relatively low percentage of death attributable to secondary malignancies was present (Table 2).
Evolution of mortality after transplantation over time
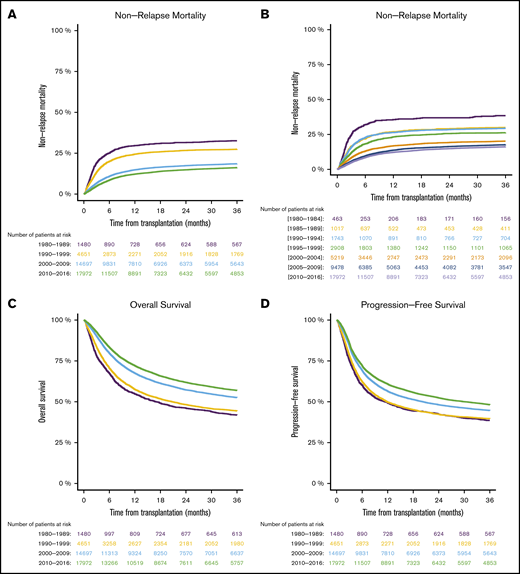
We found that NRM at 1 year after transplantation decreased over time: 29.7% from 1980 through 1989, 24.4% from 1990 through 1999, 14.8% from 2000 through 2009, and 12.2% from 2010 through 2016 (Figure 1A). The evolution of NRM in 5-year periods over time is visualized in Figure 1B. Supplemental Table 1 demonstrates that reduction of NRM over time was present in different transplant-recipient age groups. The reduced NRM translated into decreased overall mortality after transplantation. Overall survival at 1 year after allo-SCT was 54.8% from 1980 through 1989, 57.6% from 1990 through 1999, 67.5% % from 2000 through 2009, and 72.1% from 2010 through 2016 (Figure 1C). Progression-free survival, considering the underlying malignancy, was also higher in the more recent cohorts: 49.2% from 1980 through 1989, 49.8% from 1990 through 1999, 56.4% from 2000 through 2009, and 60.8% from 2010 through 2016 (Figure 1D). In multivariate analysis, the year of transplantation (in 5-year increments) was associated with reduced NRM (P < .0001; HR [95% CI], 0.8 [0.79-0.82]), reduced overall mortality (P < .0001; HR [95% CI], 0.87 [0.86-0.88]), and improved progression-free survival (P < .0001; HR [95% CI], 0.9 [0.89-0.91]). A description of the univariate outcomes is given in Table 3. Detailed results of multivariate analyses are given in supplemental Table 2.
Outcomes in different time periods of adult patients with hematologic malignancy who underwent first allo-SCT with a transplant from a matched family donor. The periods were separated into decades (A,C-D), and NRM was separated into 5-year periods (B). NRM (A-B), overall survival (C), and progression-free survival (D).
Outcomes in different time periods of adult patients with hematologic malignancy who underwent first allo-SCT with a transplant from a matched family donor. The periods were separated into decades (A,C-D), and NRM was separated into 5-year periods (B). NRM (A-B), overall survival (C), and progression-free survival (D).
Relapse and GVHD
The incidence of relapse of the primary malignancy increased slightly in univariate description over time (Table 3). However, after adjustment for confounders in multivariate analyses, we found a different picture. The incidence of relapse decreased slightly but significantly over time in the multivariate analysis (P < .0001; HR [95% CI], 0.96 [0.95-0.98] for 5-year increments). This discrepancy between univariate and multivariate analyses probably reflects the increasing number of high-risk patients over time.
The incidence and severity of acute GVHD decreased over time (Table 3). In multivariate analysis, the year of transplantation (5-year increments) was associated with reduced acute GVHD grades 2 to 4 (P < .0001; HR [95% CI], 0.92 [0.91-0.94]) and grades 3 to 4 (P < .0001; HR [95% CI], 0.9 [0.88-0.93]). Table 3 shows a higher incidence of chronic GVHD in the more recent cohorts. However, in the multivariate analysis, the year of transplantation (in 5 years increments) was associated with decreased chronic GVHD of all grades (P = .0003; HR [95% CI], 0.97 [0.96-0.99]), but not with extensive chronic GVHD (P = .31; HR [95% CI], 1.01 [0.99-1.03]).
Matched-pairs analysis
We wanted to better quantify the improvement in nonrelapse mortality by comparing the mortality of patients with similar characteristics in the 1990s vs today. We therefore performed a matched-pairs analysis, taking into consideration the type of malignancy, remission status before transplantation, age, and sex. In the 1990s cohort we identified 3718 patients who could be matched 1:1 to 3718 patients who underwent transplantation from 2013 through 2016. Additional patient characteristics in the matched cohorts are given in Table 4. Again, the most prominent differences between the cohorts included the use of peripheral blood stem cells, in vivo T-cell depletion, and reduced-intensity conditioning in the more recent cohort.
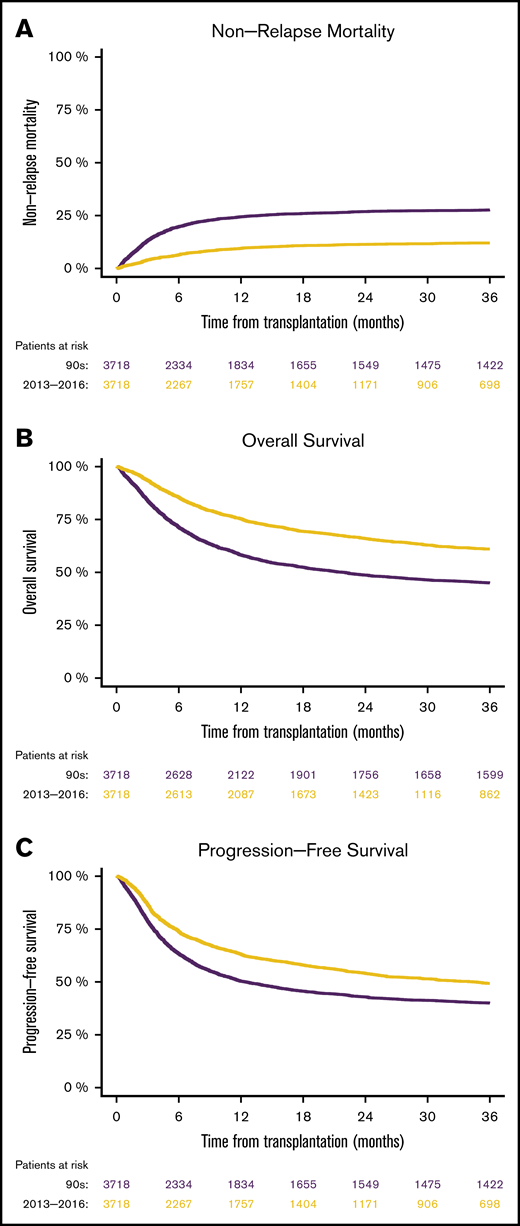
In matched patients, we found that NRM at 1 year was 24.4% from 1990 through 1999, compared with 9.5% from 2013 through 2016 (P < .0001; HR [95% CI], 0.39 [0.34-0.43]; Figure 2A). Consequently, overall survival at 1 year was superior in the 2013 through 2016 cohort (75.3%), compared with the 1990 through 1999 cohort (58.2%; P < .0001; HR [95% CI], 0.6 [0.56-0.64]; Figure 2B). Survival free from progression of the primary malignancy was also higher in the more recent cohort (63% vs 50.3%; HR [95% CI], 0.73 [0.68-0.78], P < .0001; Figure 2C). The relapse rates in the 2 different cohorts were not significantly different (HR [95% CI], 1.02 [0.95-1.11]; P = .55).
Matched-pairs analyses of mortality in patients after allo-SCTs performed from 1990 through 1999 vs those performed from 2013 through 2016. NRM (A), overall survival (B), and progression-free survival (C).
Matched-pairs analyses of mortality in patients after allo-SCTs performed from 1990 through 1999 vs those performed from 2013 through 2016. NRM (A), overall survival (B), and progression-free survival (C).
Discussion
Since the 1980s, we have observed a significant reduction in NRM with allo-SCT from HLA-matched sibling donors. The results of the multivariate analysis, adjusted for known risk factors for NRM, suggest that improvements in patient management rather than patient characteristics were the major factors driving the decreased transplantation-related mortality in modern times. We found that NRM was largely related to posttransplantation infections, severe GVHD, and organ toxicity. Although we found improvements in each of these categories, the study design did not allow conclusions to be drawn regarding the direct causes of the observed effects.
We found a continuous improvement in NRM over time. To better quantify and visualize the improvements that have been made, we performed a matched-pairs analysis of patients who underwent transplantation from 1990 through 1999, compared with a current cohort. We found that current patients were 62% less likely to die of NRM after allo-SCT than comparable patients in the 1990s. NRM decreased from 27% to 11% in patients in the 1990s and current patients, respectively, which led to an absolute reduction of 16%. Numerous advances in different areas related to allo-SCT may have contributed to improved outcomes. Important aspects involve improvements in supportive care (intensive care medicine, infectious complications, and GVHD management) and conditioning regimens. The survival of patients admitted to intensive care wards after allo-SCT has improved significantly, thanks to global improvements in intensive care medicine and to specific management aspects, such as earlier referral and earlier use of noninvasive ventilation techniques in patients with pulmonary complications after allo-SCT.7,8 A contributory reason for improved infection-related mortality in transplant recipients in recent years is the availability of more potent antiviral and antifungal drugs.3 In addition, the more frequent use of peripheral blood as a donor stem cell source is associated with shorter periods of neutropenia compared with bone marrow, potentially leading to a reduced infection risk.9 Furthermore, more sensitive laboratory tests, as well as improved imaging techniques have been developed during this period, enabling earlier diagnosis and more effective treatment of infections. Important modifications in GVHD prevention include a higher frequency of the use of in vivo T-cell depletion as prophylaxis, leading to a reduced incidence of GVHD.10 Furthermore, there is a trend toward the use of lower steroid doses in less severe cases of GVHD, potentially leading to better immunity.11,12 Finally, an increased use of less toxic conditioning regimens (reduced-intensity conditioning) in the recent cohort may have had favorable effects on organ toxicities, including multiorgan failure, and on infections as well as on GVHD.13,14
Our current results must be put into perspective with those of existing related publications. Single center analyses from the Fred Hutchinson Cancer Research Center15,16 and the Karolinska Institute17 have demonstrated reduced NRM over longer periods of time. These studies have analyzed mortality after family donor transplantation as well as unrelated donor transplantation. Our current results confirm these observations in a large international data set of sibling donor transplant recipients with different patient and treatment characteristics. The Center for International Blood and Marrow Transplant Research has published analyses of patients with acute myeloid leukemia, who underwent allo-SCT after myeloablative conditioning.18 In this specific patient population, the NRM decreased between 1985 and 2004. These data were later confirmed by the EBMT in patients with acute myeloid leukemia19 and with acute lymphoblastic leukemia.20 A recent publication, also from the EBMT, analyzed survival in a large data set of adult patients with hematological malignancies who underwent allo-SCT from 2001 through 2015, including all donor types and stem cell sources.21 The researchers found a moderate improvement in overall survival during the study period (from 46.3% to 50.5% at 3 years after transplantation) that was consistent in the different donor type groups. The most prominent differences in study design compared with our present study are (1) the different durations of the study periods (2001 through 2015 vs 1980 through 2016), which enabled investigation of long-term changes, and (2) the inclusion criteria, which allowed for the use of all donor types and stem cell sources in the recent EBMT study vs our focus on matched sibling donors with peripheral blood or bone marrow as the donor graft source. The focus on sibling donors enabled us to analyze NRM independent from the influence of high-resolution HLA typing quality, which improved considerably over time. However, based on our results, only limited conclusions can be drawn regarding the use of transplants from other than matched sibling donors. Another limitation of our study is the absence of data on genetic risk stratification of the underlying malignancy in the earlier cohorts. The probability of relapse after transplantation depends on certain genetic risk factors present in the hematologic malignancy,22,23 making it challenging to interpret data on relapse incidence without this information.
The current analysis has several clinical implications. One aspect is that the present data can be used as a benchmark for quality management purposes in individual transplantation centers. In addition, these results can be used to guide treatment decisions in patients with hematologic malignancies and other diseases. The choice of treatment is specifically a matter of current debate, because several novel therapies (such as chimeric antigen receptor T cells, bispecific antibodies, checkpoint inhibitors, and small molecules) for hematologic malignancies have already gained market access or are expected to be available in the near future, thereby coming in competition with allo-SCT for similar indications. Short-term efficacy and safety data of novel therapies are encouraging, but the availability of long-term follow-up data is a significant advantage in favor of allo-SCT. Our study delivers reliable mortality-risk data of allo-SCT over a longer period. Dissemination of knowledge in the medical community on improved stem cell transplantation outcomes may influence referral of patients to this life-saving procedure in light of the increasing number of alternative treatment options for hematologic malignancies.
A future challenge is how to achieve further improvement of survival after allo-SCT. A relevant factor is reduction of relapse rates by including novel treatment approaches and therapies in posttransplantation therapeutic strategies. Further reduction of NRM may be achieved by refining the use of risk-adapted transplantation strategies based on combining already validated prediction tools24-26 or by merging them with novel prediction tools based on molecular testing or on machine learning algorithms.
Taken together, the results of our analyses showed that NRM has decreased significantly over time. Advances in intensive care medicine and treatments of infectious disease and GVHD and reductions in the intensity of conditioning regimens are the likely contributors to better outcomes after allo-SCT.
Publication-related data are available by e-mail request to the corresponding author Olaf Penack (olaf.penack@charite.de).
Acknowledgments
This work was supported by grants from José Carreras Leukämie-Stiftung (3R/2019), Deutsche Krebshilfe (70113519), and Deutsche Forschungsgemeinschaft (PE 1450/7-1) (O.P.).
Authorship
Contribution: All authors contributed substantially to the manuscript; O.P., C.P., H.S., C.K., Z.P., and G.W.B. contributed to the conception and design of the study and to analysis or interpretation of the data; O.P. drafted the article; all authors critically revised and approved the manuscript; and all authors are accountable for all aspects of the work by ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved.
Conflict-of-interest disclosure: Between 2017 and 2020, H.S. participated in advisory boards for Incyte (2018) and Janssen and Novartis (2020); received speaker’s fees from Jazz Pharmaceuticals (2017), Novartis and Incyte (2018), and Incyte, Jazz Pharmaceuticals, and Takeda (2019); received travel grants from EBMT (2017), EBMT, Celgene, and Abbvie (2018), EBMT and Incyte (2019), and EBMT and Gilead (2020); and received research funding from Novartis for an investigator-initiated study (2020). The remaining authors declare no competing financial interests.
Correspondence: Olaf Penack, Department of Hematology, Oncology, and Tumorimmunology, Charité Universitätsmedizin Berlin, Campus Virchow Clinic, Augustenburger Platz 1, 13353 Berlin, Germany; e-mail: olaf.penack@charite.de.
References
Author notes
The full-text version of this article contains a data supplement.