Key Points
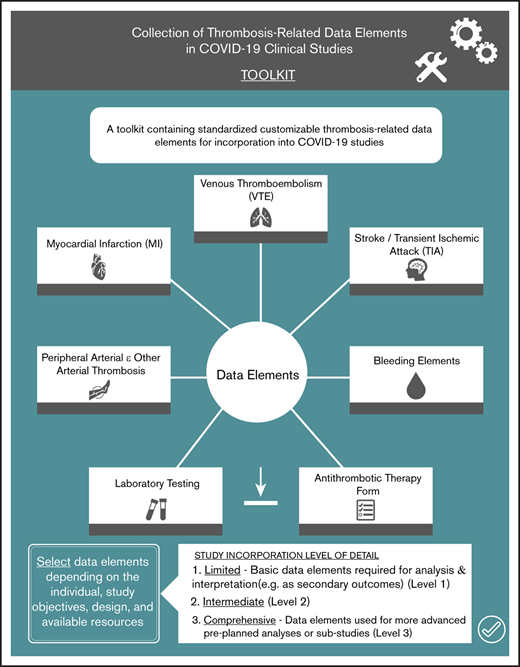
This toolkit contains standardized customizable thrombosis-related data elements for incorporation into COVID-19 studies.
Standardization can enhance the quality of data collection, create efficiency, and facilitate comparison of results and pooling of data sets.
Abstract
Thrombosis has emerged as an important complication of coronavirus disease 2019 (COVID-19), particularly among individuals with severe illness. However, the precise incidence of thrombotic events remains uncertain due to differences in study design, patient populations, outcome ascertainment, event definitions, and reporting. In an effort to overcome some of these challenges and promote standardized data collection and reporting in clinical studies, the American Society of Hematology Research Collaborative COVID-19 Non-Malignant Hematology Task Force, in collaboration with the International Society on Thrombosis and Haemostasis COVID-19 Task Force, developed sets of data elements in the following domains: venous thromboembolism, myocardial infarction, stroke/transient ischemic attack, peripheral arterial thrombosis, bleeding, laboratory investigations, and antithrombotic therapy. Data elements in each of these domains were developed with 3 levels of detail to facilitate their incorporation into studies evaluating a range of interventions and outcomes. Previously published data elements were included where possible. The use of standardized variables in a range of clinical studies can enhance the quality of data collection, create efficiency, enhance comparison of results across studies, and facilitate future pooling of data sets.
Introduction
The global fight against coronavirus disease 2019 (COVID-19) has galvanized the global research community into action, with >3600 registered clinical studies and almost 60 000 publications to date listed on PubMed (accessed October 18, 2020).
Coagulopathy and an apparent increased risk of both venous and arterial thrombosis have emerged as prominent features of severe COVID-19.1 COVID-19 coagulopathy seems distinct from the hyperfibrinolytic consumptive disseminated intravascular coagulation typical of non–COVID 19 sepsis. It is characterized by markedly elevated D-dimer levels, normal or increased fibrinogen levels, and normal prothrombin time, partial thromboplastin time, and platelet counts. A high incidence of venous thromboembolism (VTE) has been reported in some cohort studies, particularly among hospitalized patients (3%) and those admitted to intensive care units (11%-69%).2-9 The wide range of event rates, which precludes precise estimates of incidence, likely reflects differences in study design, patient populations, outcome ascertainment, and event definitions. Furthermore, results are difficult to compare across studies due to inconsistent reporting about the nature and location of VTE, the clinical context in which it was diagnosed, and the use of antithrombotic therapy.
For example, some studies did not report the location of the VTE (ie, distal vs proximal deep vein thrombosis, subsegmental vs more proximal pulmonary embolism), which has prognostic implications.10,11 Furthermore, diagnostic strategies for VTE were variable, and there was inconsistent reporting of whether VTE was diagnosed based on the presence of clinical signs or symptoms, incidental imaging findings, or systematic screening of asymptomatic patients. Finally, standardized collection of information on antithrombotic therapy use (eg, anticoagulants, antiplatelet therapies) and dosing preceding thrombotic events is crucial for understanding the magnitude of the thrombotic risk, which is affected by these agents administered for the prevention and treatment of thrombotic disease in at-risk patients (eg, VTE, atrial fibrillation, mechanical heart valve, coronary artery disease, stroke, peripheral arterial disease). In addition to evidence suggesting a high risk of VTE, the rate of ischemic stroke seems to be higher among patients with COVID-19 who are hospitalized or have emergency department visits compared with similar patients with influenza A/B.12
Methodologic limitations inherent to observational study designs (eg, selection and ascertainment bias) preclude firm conclusions about the degree of risk and incidence of thrombosis in patients with COVID-19. As a result, substantial uncertainty remains regarding the incidence, risk factors, and natural history of different types of thrombotic complications in patients with COVID-19, which has limited the clinical utility of these findings and impaired the ability to provide clear guidance to physicians.13,14
Although clinical trials assessing antithrombotic strategies in patients with COVID-19 are underway, studies evaluating other treatments (eg, antiviral therapies, immunomodulatory drugs, convalescent plasma) offer additional opportunities to further characterize the risk of thrombosis, predictors, and prognosis of thrombotic complications in patients with COVID-19. Harmonizing data elements with standardized collection and reporting of the type and location of events, the method of diagnosis (including the presence of symptoms), the use of antithrombotic therapies (including anticoagulant and antiplatelet treatments) at the time of thrombotic events, and the presence of preceding risk factors will help achieve this goal.
We aim to support and enhance the collection and reporting of data elements involved in key thrombosis (and related) end point events in COVID-19 studies by providing investigators with a set of clinical and biological variables with a range of details ranging from limited (eg, for capturing events as secondary outcomes) to comprehensive (eg, for more in-depth analyses) along with associated variable definitions. Our intent was not to be overly prescriptive but to allow for the flexibility needed by global investigators conducting clinical research. These data elements are a first step in developing a common language for capturing thrombosis and related events in clinical research in COVID-19.
Methods
Writing committee composition
The American Society of Hematology Research Collaborative (ASHRC) convened a COVID-19 Non-Malignant Hematology Task Force to develop and implement projects related to hematologic complications of COVID-19. In collaboration with the International Society on Thrombosis and Haemostasis (ISTH) COVID-19 Task Force, the objective of the initiative described herein was to provide uniform definitions of thrombosis, bleeding, and laboratory data elements for incorporation into COVID-19 clinical studies addressing a diverse range of interventions. The writing committee with international representation consisted of 8 individuals with expertise in VTE, cardiovascular medicine, hematology, clinical research, epidemiology, outcomes assessment, medical informatics, health information management, and health care services research. The manuscript was drafted by the writing committee and reviewed by members of the ASHRC COVID-19 Non-Malignant Hematology Task Force and ISTH COVID-19 Task Force.
Data elements and definitions
The writing panel focused on variables that were inconsistently reported in initial studies about thrombosis in patients with COVID-19.
We identified data elements and definitions in the following domains: (1) VTE; (2) myocardial infarction; (3) stroke; (4) peripheral arterial thromboembolism; (5) bleeding; (6) antithrombotic therapy; and (7) basic laboratory investigations. The writing committee recognized that individual study objectives, interventions, and available resources will influence the desired level of detail about thrombosis-related outcomes. Therefore, to facilitate incorporation of data element collection and reporting by investigators, we identified the following 3 levels of detail: (1) limited; (2) intermediate; and (3) comprehensive. For example, the limited data set contains basic data elements required for analysis and interpretation (eg, as secondary outcomes), whereas the comprehensive data set contains data elements that could be used for more advanced preplanned analyses or substudies.
For VTE, bleeding, antithrombotic therapy, and laboratory data points, the writing panel selected and/or adapted data elements from the recently released ISTH Common Data Elements for VTE project, a large initiative aimed at harmonizing data elements collected and reported in VTE-related clinical trials.15 A draft document including the sets of variables was reviewed by the members of the ASHRC COVID-19 Non-Malignant Hematology Task Force and the ISTH COVID-19 Task Force. All comments were considered by the group, and final decisions on data elements to be retained were made by consensus.
Results
Discussion
The goal of this initiative was to maximize high-quality collection of COVID-19–related thrombosis outcomes by providing a customizable data collection tool for use in clinical studies on COVID-19 that may focus on areas other than thrombosis. Depending on the individual study objectives, design, and available resources, investigators can select from 3 levels of detail in the following domains: VTE, myocardial infarction, stroke, peripheral arterial and other arterial thromboembolism, antithrombotic therapy, bleeding, and laboratory tests. This approach is designed to create efficiency in study design and implementation by providing a pre-established, standardized set of variables vetted by experts in thrombosis and hemostasis and clinical trial development. Where available, we incorporated definitions that are widely accepted in the thrombosis and hemostasis community.16-18 We also used common data elements from the ISTH Common Data Elements for VTE Research project and adapted other previously published common data elements.15,19,20
A specific challenge with the COVID-19 pandemic has been the unprecedented need for rapid development and implementation of clinical studies worldwide, which has resulted in multiple small studies addressing similar research questions without the benefit of large-scale communication and collaboration. Logistical challenges and declining infection rates in some areas may impair the ability to execute and complete clinical trials. The use of standardized variables can leverage data collection efforts across studies for potential future pooled analyses, including individual participant data meta-analyses. Working together, ASH and ISTH have developed a multimodal promotional strategy including direct e-mails to members, announcements on social media, resources posted on their Web sites, and direct outreach to other professional societies and funding agencies.
Our initiative has some limitations. Given the need to provide urgent guidance to investigators, we did not conduct a systematic review or use conventional consensus methods (eg, Delphi). However, the panel comprised physicians and researchers with experience in clinical trials and observational studies on venous and arterial thrombosis and data harmonization efforts. Although the variables were reviewed and selected by the ASHRC COVID-19 Non-Malignant Hematology Task Force and ISTH COVID-19 Task Force with a view to providing flexible options for a range of non-thrombosis clinical trials, the data collection sets may not be suitable for every study depending on the specific objectives or patient population. We also acknowledge that with the rapid evolution of the COVID-19 pandemic, the current set of proposed variables may require modification to incorporate new developments or emerging knowledge of the disease. This initiative can therefore also serve as an adaptable platform to support future research efforts through an iterative review process.
Requests for data sharing may be submitted to the corresponding author (Grégoire Le Gal; e-mail: glegal@ohri.ca).
Acknowledgments
The authors thank Stephanie Lee (President, ASH), Cary Clark (Director of Programs and Education, ISTH), Robert Plovnick (Chief Medical Quality Officer, American Society of Hematology and ASHRC), Lacey Schmeidler (Program Manager, ISTH), and Emily Tucker (Senior Manager, Evidence Development, ASHRC) for their guidance and support through this initiative. They also thank additional members of the ASHRC COVID-19 Non-Malignant Hematology Task Force (Mark Crowther, Julie Kanter, Alice Ma, Donna Neuberg, Adam Cuker Jean Connors, Neil Zakai, Lisa Baumann Kreuziger, and Maureen Achebe) and the ISTH COVID-19 Task Force (Claire McLintock, Gabriela Cesarman Maus, Roberta Gualtierotti, Nigel Key, Nicola Mutch, Frits Rosendaal, and Wolfram Ruf) for their review of this work.
G.L.G. is supported by an Early Researcher Award from the Province of Ontario, a Mid-Career Investigator Award from the Heart and Stroke Foundation of Ontario, and a Research Chair on the Diagnosis of Venous Thromboembolism, Department of Medicine, University of Ottawa. The contribution of N.J.L. was supported by the CanVECTOR and INVENT research networks.
Authorship
Contribution: D.M.S., G.D.B., N.J.L., A.L., S.M., L.S., W.A.W., and G.L.G. developed the variables; D.M.S. and G.L.G. wrote the manuscript; and L.S., A.L., G.D.B., N.J.L., W.A.W., and S.M. revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Grégoire Le Gal, The Ottawa Hospital, General Campus, Box 201A, 501 Smyth Rd, Ottawa, ON K1H 8L6, Canada; e-mail: glegal@ohri.ca.
References
Author notes
The full-text version of this article contains a data supplement.