Key Points
Epigenetic therapy after allo-HSCT with panobinostat alone and in combination with low-dose decitabine is feasible in poor-risk AML.
Results did not suggest a synergistic or additive effect of combining panobinostat with decitabine.
Abstract
Outcome after allogeneic hematopoietic stem cell transplantation (allo-HSCT) is adversely affected by relapse to a considerable degree. To exploit the graft-versus-leukemia effect more effectively, we assessed the feasibility of early initiation of epigenetic therapy with panobinostat and decitabine after allo-HSCT and before donor lymphocyte infusion (DLI) in poor-risk patients with acute myeloid leukemia (AML) or refractory anemia with excess blasts with International Prognostic Scoring System score ≥1.5. A total of 140 poor-risk patients with AML aged 18 to 70 years were registered, and 110 proceeded to allo-HSCT. Three dose levels were evaluated for dose-limiting toxicities, including panobinostat monotherapy 20 mg at days 1, 4, 8, and 11 of a 4-week cycle (PNB mono group) and panobinostat combined with either decitabine 20 mg/m2 (PNB/DAC20 group) or decitabine 10 mg/m2 (PNB/DAC10 group) at days 1 to 3 of every 4-week cycle. After phase 1, the study continued as phase 2, focusing on completion of protocol treatment and treatment outcome. PNB mono and PNB/DAC10 were feasible, whereas PNB/DAC20 was not related to prolonged cytopenia. Sixty of 110 patients who underwent transplantation were eligible to receive their first DLI within 115 days after allo-HSCT. Grade 3 and 4 adverse events related to panobinostat and decitabine were observed in 23 (26%) of the 87 patients, and they received epigenetic therapy. Cumulative incidence of relapse was 35% (standard error [SE] 5), and overall survival and progression-free survival at 24 months were 50% (SE 5) and 49% (SE 5). Post–allo-HSCT epigenetic therapy with panobinostat alone or in combination with low-dose decitabine is feasible and is associated with a relatively low relapse rate. The trial was registered at the European Clinical Trial Registry, https://www.clinicaltrialsregister.eu, as ECT2012-003344-74.
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is the preferred treatment to consolidate remission in (very) poor-risk patients with acute myeloid leukemia (AML).1-3 The graft-versus-leukemia (GVL) effect of allo-HSCT reduces relapse in a similar, relative degree in all subcategories of AML.4,5 Despite the presence of a strong GVL effect, the absolute incidence of relapse in poor-risk patients with AML remains considerable.1 To optimize the immunotherapeutic effect of GVL, several phase 1 and 2 trials were conducted to explore the use of hypomethylating agents after allo-HSCT, with the goal of restoring epigenetic deregulation of residual leukemic cells and enhancing allogeneic immunotherapy.6-11 In addition, the feasibility of the histone deacetylation inhibitor panobinostat when given after allo-HSCT has been shown.12 Combining hypomethylating drugs with a histone deacetylation inhibitor has been investigated in vitro13-16 and in vivo17-19 and may exert additive or synergistic effects; however, the feasibility and efficacy of their combination in patients with AML is currently unknown. In this multicenter phase 1/2 Dutch-Belgian Haemato-Oncology Foundation for Adults (HOVON) trial, we addressed the feasibility of early epigenetic therapy by panobinostat alone and in combination with decitabine before donor lymphocyte infusion (DLI) in recipients of allo-HSCT.
Methods
Patient selection and materials
All patients with AML were registered after diagnosis and classification as poor risk, before the start of the second cycle of induction chemotherapy. The study was open for patient accrual from November 2013 through August 2017. Poor- and very-poor-risk patients aged 18 to 70 years who had a diagnosis of AML or refractory anemia with excess blasts with International Prognostic Scoring System score ≥1.5, according to the latest (2015) HOVON-AML risk classification, were eligible (supplemental Appendix, details of the HOVON 116 study, section 1), if the intention was to perform allo-HSCT as consolidation therapy after 2 cycles of chemotherapy. A donor search started immediately after the patient was registered, with the purpose of performing a transplantation within 6 to 8 weeks after the second cycle of induction chemotherapy with either a matched sibling or a matched unrelated donor. At the time of allo-HSCT, patients had to fulfill a second set of eligibility criteria, including disease response (<10% blasts at 3 and/or 4 weeks after the start of cycle 2) and HLA-compatible donor availability (≥7 of 8 HLA-matched unrelated donor or fully matched sibling donor). A detailed description of the inclusion and exclusion criteria of the study are presented in the supplemental Appendix (details of the HOVON 116 study, sections 2 and 3). Minimal residual disease (MRD) was assessed in bone marrow samples, and flow cytometry analysis was performed in a 2-step procedure, as previously described.20,21 MRD samples were obtained after cycle 2 and before allo-HSCT. The trial was approved by the ethics committees of the participating institutions and was conducted in accordance with the Declaration of Helsinki. All participants gave written informed consent.
Study design
The study was designed as a prospective multicenter phase 1/2 feasibility study. Patients were registered during induction chemotherapy for newly diagnosed AML or myelodysplasia syndrome. Subsequently, they were required to fulfill a second set of eligibility criteria just before allo-HSCT. The study started as a phase 1 study exploring 3 dose levels in 29 patients (part 1). After the feasibility of 2 of the 3 dose levels was demonstrated, the study continued as a phase 2 feasibility study focusing on treatment outcome parameters and completion of the protocol treatment (part 2).
Part 1.
In the first part of the study, the feasibility of epigenetic combination therapy was assessed. Only very-poor-risk patients with AML were included in this part of the study. Dose levels consisted of panobinostat monotherapy (20 mg) alone at days 1, 4, 8, and 11 of a 4-week cycle (PNB mono) and in combination with decitabine (PNB/DAC20) at a dose of 20 or 10 mg/m2 at days 1 to 3 of every 4-week cycle (PNB/DAC10). PNB mono and PNB/DAC10 appeared feasible, and those treatment groups therefore included more patients to address the questions in part 2 of the study.
The primary end point of part 1 was feasibility, as defined by the number of dose-limiting toxicities (DLTs) during the first cycle of epigenetic therapy (<4 of 10 patients). DLTs included grade 4 nonhematological toxicity, treatment-related mortality, and hematological toxicity between cycles 1 and 2 of epigenetic therapy, which resulted in delay of the start of cycle 2 after day 35 because of the transplantation (supplemental Figure 1; supplemental Appendix, details of the HOVON 116 study, section 5).
Part 2.
Part 2 of the study continued with inclusion of patients in the feasible dose levels of the PNB mono and PNB/DAC10 groups. This part of the study focused on the completion of protocol treatment within the defined time frame of 115 days up to the first DLI, without the occurrence of adverse events (AEs) and/or acute graft-versus-host disease (GVHD). The inclusion and exclusion criteria for patients to continue with epigenetic therapy are noted in the supplemental Appendix (details of the HOVON 116 study, section 4). AEs were graded using the Common Terminology Criteria for Adverse Events, version 4.0.22 Acute and chronic GVHD was defined according to the latest proposal of the recent National Institutes of Health Consensus Conference.23 Phase 2 of the study was performed as a Simon-2 stage design for DLT-feasible dose levels separately, including interim analyses. Secondary end points included survival analyses consisting of overall survival (OS) from the date of allo-HSCT, cumulative incidence of relapse (CIR), nonrelapse mortality (NRM), and progression-free survival (PFS) from the date of allo-HSCT.
Treatment protocol
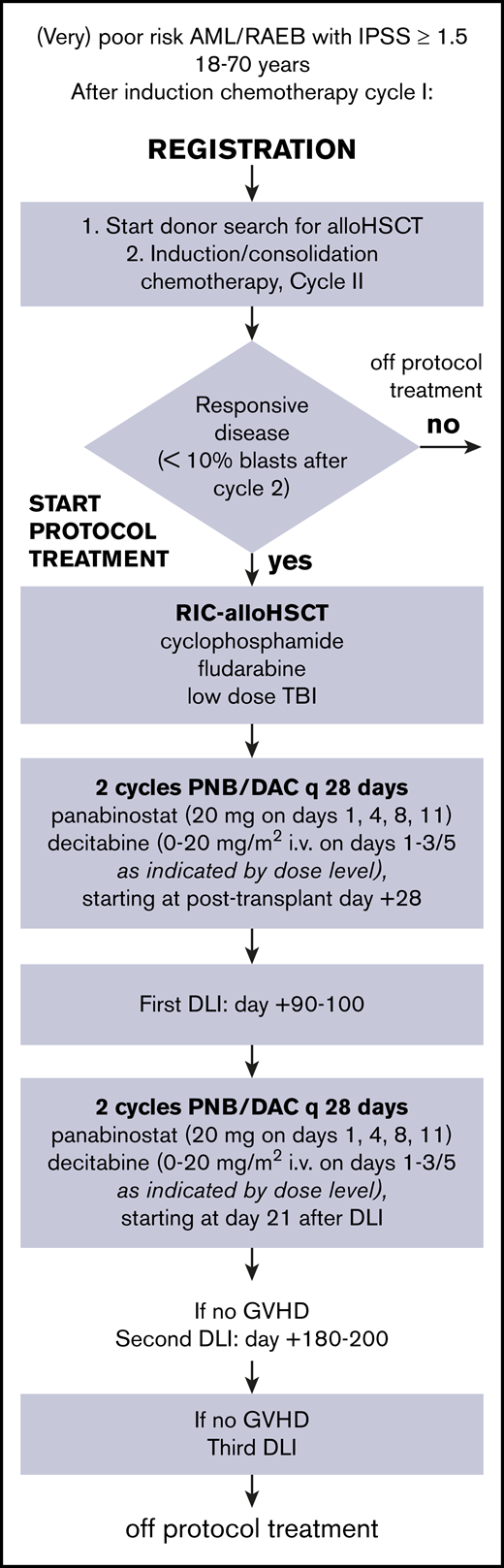
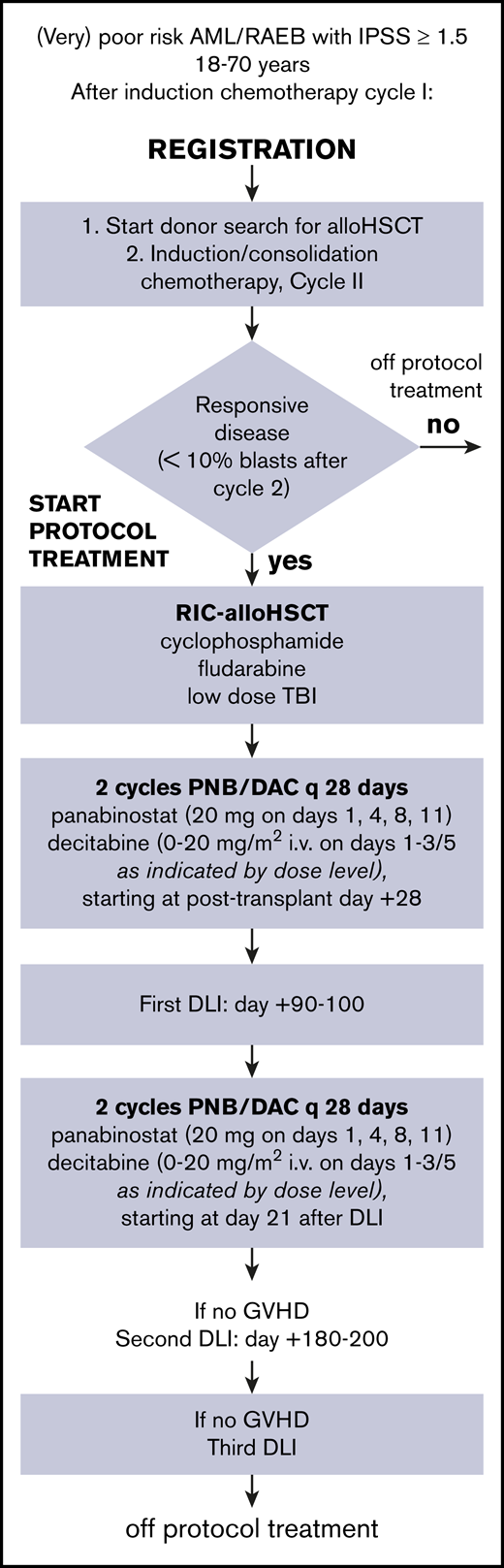
The treatment protocol is summarized in Figure 1. All patients received 2 cycles of induction chemotherapy, regardless of response to cycle 1. Patients received idarubicin 12 mg/m2 at days 1 to 3 and cytarabine 200 mg/m2 at days 1 to 7 in cycle 1, followed by daunorubicin 60 mg/m2 at days 1, 3, and 5 and 1000 mg/m2 at days 1 to 6 in cycle 2. T-cell–replete allogeneic transplantation was performed after conditioning with intravenous cyclophosphamide 14.5 mg/kg per day at days −6 and −5, fludarabine 30 mg/m2 per day at days −6 to −2, and 2-Gy total body radiation at day −1. At days +3 and +4, cyclophosphamide (50 mg/kg per day IV) was given for prevention of GVHD and to allow for early initiation of epigenetic therapy, followed by 3 mg/kg per day cyclosporine at days +5 to +70, based on through levels. Patients were assigned to 4 cycles of epigenetic therapy, interspersed with the first DLI at day +90 to +100 and the second one at day +180 to +200 after allo-HSCT. Cyclosporine was discontinued before the first DLI. The first cycle started at day 28 after allo-HSCT or as soon as possible thereafter, when the patient met the criteria (supplemental Appendix, details of the HOVON 116 study, section 4). After the first DLI, patients received cycle 3 and 4 of epigenetic therapy. Cycle 3 started at day 21 after DLI, and cycle 4 was given at day 49, or as soon as possible after hematological recovery.
Scheme of HOVON 116 study from registration to cessation of protocol treatment. DAC, decitabine; IPSS, International Prognostic Scoring System; PNB, panobinostat; RAEB, refractory anemia with excess blasts; RIC, reduced-intensity conditioning.
Scheme of HOVON 116 study from registration to cessation of protocol treatment. DAC, decitabine; IPSS, International Prognostic Scoring System; PNB, panobinostat; RAEB, refractory anemia with excess blasts; RIC, reduced-intensity conditioning.
Statistical methods
OS, CIR, NRM, and PFS were analyzed using actuarial estimates and the Kaplan-Meier method. The event for OS consisted of death from any cause, and the patients were censored at the date of last contact. The events for PFS were death in remission and relapse. The cumulative risks of relapse and NRM over time were calculated as competing risks with actuarial methods, whereas patients alive and continuing in complete response 1 (CR1) were censored at the date of last contact. The starting point of all survival curves is the date of transplantation, if not stated otherwise. All P values were based on log-rank tests.
Results
Patient and transplant characteristics
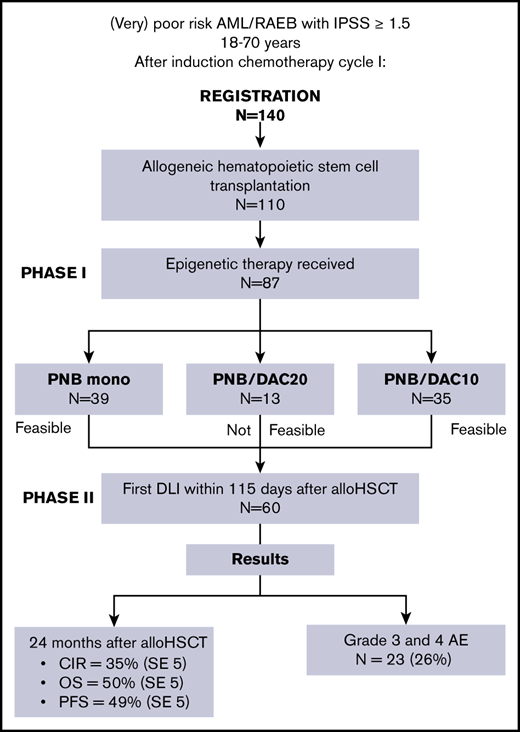
A total of 140 (very) poor-risk patients with AML were registered in this study during induction chemotherapy for AML or myelodysplasia syndrome; 110 patients underwent an allo-HSCT according to protocol, with either a matched sibling or a matched unrelated donor. The diagnosis of poor-prognosis AML was revised in 1 patient after registration. As a result, this patient was removed from all additional analyses. The reason for exclusion of the remaining 29 patients included refractory AML (n = 8), no sibling or unrelated donor available (n = 7), refusal (n = 4), death (n = 3), and other (n = 7). Patient characteristics are presented in Table 1. Median age at diagnosis was 59 years (range, 18-71). World Health Organization (WHO) performance status was 0 to 1 in 92 patients. Thirty-five patients were refractory to the first cycle of chemotherapy. Of them, 31 and 4 patients achieved CR and partial response, respectively, upon the second cycle of induction chemotherapy. According to the HOVON/SAKK (Swiss Group for Clinical Cancer Research) risk classification (supplemental Appendix, details of the HOVON 116 study, section 1), 77 patients were classified as very poor risk. Transplant characteristics are depicted in Table 2. All patients received 2 cycles of induction chemotherapy. The median time from evaluation of the second cycle to allo-HSCT was 16 days (range, 1-66), and the median time from diagnosis to allo-HSCT was 110 days (range, 56-205). Forty-one patients received a graft from a matched sibling donor, 61 from a fully matched unrelated donor, and 8 from a mismatched unrelated donor. One patient received a bone marrow graft from a sibling donor. The median follow-up of patients who lived was 23 months. Sixty-seven patients exhibited an EBMT (European Society for Blood and Marrow Transplantation) score of ≥3 points.24
Part 1: feasibility of panobinostat alone and in combination with decitabine
Analyses were performed on data from the first 10 patients in the PNB mono and PNB/DAC10 groups. At interim analysis, 1 of 9 patients in the in PNB mono group experienced DLT. Four of 10 patients receiving PNB/DAC20 experienced DLT, consisting of prolonged cytopenia. Consequently, PNB/DAC20 was not considered feasible. The combination of panobinostat and decitabine was further evaluated in the PNB/DAC10 group (10 mg/m2 of decitabine). Only 1 DLT was observed in the first 10 patients in the group, again because of prolonged cytopenia. Subsequently, the study was expanded, according to design, by inclusion of another 55 patients in the PNB mono and PNB/DAC10 groups. Of note, in total, 13 patients were included in the PNB/DAC20 group because of ongoing inclusion of patients during the interim analysis (Figure 1).
Part 2: completion of protocol treatment and secondary end points
In the second part of the study, the completion of protocol treatment up to the first DLI and outcome was evaluated. Eighty-seven of 110 patients who underwent transplantation were eligible for epigenetic therapy and received the first cycle (Figure 1). The reasons for withdrawal of the epigenetic drugs were GVHD (n = 11), thrombocytopenia (n = 3), renal dysfunction (n = 3), death (n = 2), disease progression (n = 2), liver dysfunction (n = 1), and start of the first cycle beyond day 35 after allo-HSCT (n = 1). Of 110 patients who received allo-HSCT, 60 (55%) were eligible to receive DLI within 115 days. In total, 63 of 75 (84%) patients who received a second cycle of epigenetic therapy received their first planned DLI. Second and third DLIs were given to 40 and 25 patients, respectively. AEs considered to be related to panobinostat and decitabine treatment are shown in Table 3. Epigenetic therapy–related grade 3 and 4 AEs were observed in 23 (26%) of the 87 patients who received epigenetic therapy. Related hematological AEs were noted in only 3 patients, consisting of 1 grade 2 and 2 grade 3 events. In general, panobinostat- and decitabine-related AEs were rapidly reversible after treatment was interrupted. Of note, AEs were not attributable to either panobinostat or decitabine when the 2 were combined.
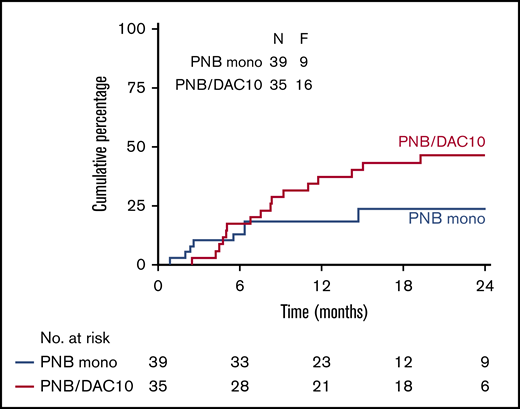
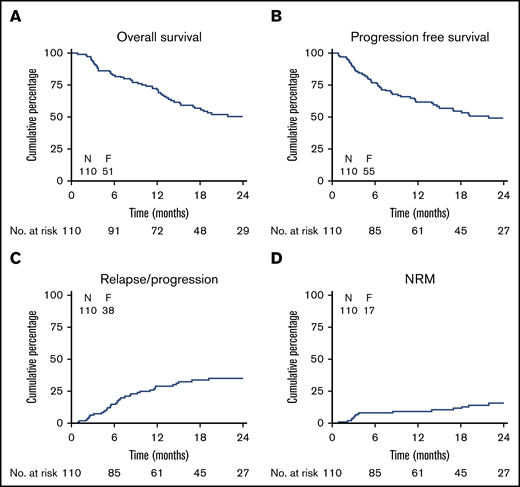
Figure 2 shows the CIR, NRM, PFS, and OS of all 110 patients who underwent transplantation. The CIR at 24 months was 35% (SE 5), and NRM at 24 months was 16% (SE 4). OS and PFS at 24 months were 50% (SE 5) and 49% (SE 5), respectively. The cumulative incidence of relapse at 24 months in the PNB mono group was 24% (SE 8), and the addition of decitabine did not improve the outcome, with a CIR at 24 months of 46% (SE 9; P = .29) in the PNB/DAC10 group (Figure 3). Although not significant, relapse was seen less often in MRD-negative than in MRD-positive patients (CIR at 24 months 35% [SE 7] vs 43% [SE 11], respectively; P = .09; supplemental Figure 3). Outcome according to the HOVON risk categories showed an OS of 63% (SE 10) and 46% (SE 6) at 24 months for poor- and very-poor-risk patients with AML, respectively (supplemental Figure 2). As expected, ineligibility for transplantation was associated with a dismal outcome; the overall survival at 12 months was only 19% (SE 8) (supplemental Figure 4).
Survival, relapse/progression and NRM. Overall (OS) and progression-free survival (PFS), relapse, and NRM Kaplan-Meier estimates of OD (A), progression-free survival (B), CIR (C), and cumulative incidence of NRM (D). F, number of failures.
Survival, relapse/progression and NRM. Overall (OS) and progression-free survival (PFS), relapse, and NRM Kaplan-Meier estimates of OD (A), progression-free survival (B), CIR (C), and cumulative incidence of NRM (D). F, number of failures.
CIR by feasible dose levels in the PNB mono and PNB/DAC10 groups. Kaplan-Meier estimates of CIR in both groups are shown.
CIR by feasible dose levels in the PNB mono and PNB/DAC10 groups. Kaplan-Meier estimates of CIR in both groups are shown.
Acute GVHD grades 3 and 4 and grades 2 to 4 at 6 months were seen in 5% (SE 2) and 23% (SE 4) of all patients, respectively, and 22% (SE 4) of the patients experienced moderate-to-severe chronic GVHD at 12 months (Figure 4). Of the 110 patients who underwent transplantation, survival was 36% (SE 5); none of the patients experienced relapse or developed acute or chronic GVHD requiring systemic therapy (supplemental Figure 5).
Acute and chronic GVHD. Acute GVHD grades 2 to 4 and grades 3 and 4 (A) and moderate/severe chronic GVHD (B). F, number of failures (ie, acute grades 2 to 4, grades 3 and 4, or moderate/severe chronic GVHD).
Acute and chronic GVHD. Acute GVHD grades 2 to 4 and grades 3 and 4 (A) and moderate/severe chronic GVHD (B). F, number of failures (ie, acute grades 2 to 4, grades 3 and 4, or moderate/severe chronic GVHD).
Discussion
Relapse after allo-HSCT remains the major cause of failure in patients with AML.1 We showed earlier that allo-HSCT reduces relapse in a similar relative degree in different subcategories of patients with AML.4,5 These findings provided evidence that allogeneic GVL is present even in very-poor-risk patients with AML, despite a higher absolute relapse rate after allo-HSCT. It provided the rationale for exploit the GVL effect more deeply by administering epigenetic therapy after allo-HSCT. Epigenetic therapy was followed by DLI, to enhance the GVL effect, given the poor antileukemic effect of panobinostat itself. In this study, we showed that post–allo-HSCT epigenetic therapy with panobinostat alone or in combination with decitabine is feasible in terms of DLTs and AEs. From intent to treat up to the first DLI, protocol treatment was administered to most patients within a strict time frame of 115 days. Outcome with respect to disease recurrence was promising, which may have been related to an enhanced GVL effect.
Grade 3 and 4 AEs related to panobinostat and decitabine were observed in 23 (21%) of the 87 patients who received epigenetic therapy. AEs related to panobinostat and/or decitabine were limited in our protocol, and hematological grade 3 and 4 disorders were noted in only 4 patients. In general, these AEs were rapidly reversible after epigenetic therapy was interrupted. In comparison, in a prospective phase 1 trial, Pusic at al9 investigated post–allo-HSCT decitabine treatment in patients with AML and reported grade 3 to 4 hematological toxicities in 75% of the patients. The difference may be explained, at least in part, by different dosages and possible drug interactions caused by the combination of GVHD-prophylaxis and post–allo-HSCT epigenetic therapy. Recently, Bug et al12 explored panobinostat after allo-HSCT as a maintenance therapy in poor-risk patients with AML. The application of panobinostat after transplantation appeared to be feasible with AEs related to panobinostat in 52% of the patients. In the present study, the strategy of posttransplant cyclophosphamide prophylaxis of GVHD, of which the feasibility and efficacy have been demonstrated elaborately before,25-34 was applied in combination with cyclosporine to enable effective GVHD prophylaxis and allow for an early start of epigenetic therapy. Thereby, the majority of allo-HSCT recipients (87 of 110; 79%) were eligible to start epigenetic therapy. In addition, cyclophosphamide appeared to be associated with limited acute and chronic GVHD and also limited NRM (16%) at 24 months. These data compare well to those in previous studies with post–allo-HSCT epigenetic therapy.6,7,9,12 Of note, in contrast to Bug et al,12 only patients transplanted in CRi or PR were included in the present study.
The early application of allo-HSCT in poor-risk patients with AML has been stressed before by Schmid et al in refractory35 and more recently in monosomal karyotype AML.36 Our study was designed to apply allo-HSCT shortly after chemotherapy. To achieve this end, patients were registered shortly after diagnosis, followed by a rapid donor search. It resulted in a median time of 16 days (range, 1-66) between evaluation after the second cycle of chemotherapy and allo-HSCT. By study design, allo-HSCT was followed by epigenetic therapy and DLI, preferably with the first DLI given by day 115 after allo-HSCT. This rather ambitious schedule was successfully achieved in 55% of patients. In previous studies exploring the hypomethylating agents decitabine and azacitidine after allo-HSCT, the percentage of patients actually receiving scheduled cycles ranged between 20% and 43%, and treatment did not include DLI.6-10 Moreover, none of these trials included patients before allo-HSCT during chemotherapy and reported results by intent to treat.
By extending the feasible dose levels, we were able to study relapse and compare relapse of patients in the PNB mono and PNB/DAC10 groups. No additive or synergistic effect of adding decitabine to panobinostat was suggested. The cumulative incidence of relapse at 24 months was 24% (SE 8) in recipients of panobinostat only, whereas the CIR was 46% (SE 9, P = .29) after combination of panobinostat and decitabine. Absence of a stronger GVL after combination therapy may be explained by an antiproliferative effect of decitabine on alloreactive T-cells, as decitabine exerts an inhibitory effect on cell proliferation by blocking DNA-synthesis, similar to, for example, cytarabine.37-40 Apart from its antiproliferative effects, decitabine may also exert an immunomodulatory effect by increasing the number of Tregs through Foxp3 demethylation.41 Either of these mechanisms could jeopardize the allogeneic GVL effect.
The results observed by Bug et al12 and us strongly warrant a prospective comparison, which is currently ongoing. Bug et al12 reported a PFS of 75% at 24 months in allo-HSCT recipients receiving panobinostat only, which is very encouraging and compares well to our results. Of note, their study included patients at a relatively late time point after allo-HSCT, leaving the possibility of patient selection by excluding patients with early relapse and those who had complications, again strongly suggesting a prospective comparison, which the 2 study groups will cooperatively perform. In addition, continuing preemptive therapy after allo-HSCT, especially in very-poor-risk patients, could be considered, given that there did not seem to be a plateau in the survival analyses. However, toxicity and efficacy would have to be weighed and evaluated.
In summary, epigenetic therapy after allo-HSCT with panobinostat alone and in combination with low-dose decitabine is feasible in poor-risk AML and may be associated with enhanced GVL. Results did not suggest a synergistic or additive effect of combining panobinostat with decitabine. Our results and those of Bug et al12 have set the stage for the current European randomized intergroup study, evaluating the efficacy of panobinostat in allo-HSCT recipients with poor-risk patients with AML (ETAL-4/HOVON145; ECT 2017-000764-15).
Original data are available by e-mail request to the corresponding author, Jan J. Cornelissen (j.cornelissen@erasmusmc.nl).
Acknowledgments
The authors thank the trial manager and the local and central data managers for collecting the patient data.
This investigator-sponsored trial was financially supported by Novartis Pharma and Janssen Pharmaceutica, and the drugs panobinostat and decitabine were provided free of charge.
Authorship
Contribution: J.J.C., E.M., M.v.G., G.J.O., and Y.v.N. designed the study; B.K., Y.v.N., and J.J.C. were involved in analyzing and interpreting the data and writing this report; and all authors provided study materials, were involved in the collection and assembly of the clinical data, and reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jan J. Cornelissen, Department of Hematology, Erasmus University Medical Center Cancer Institute, Wytemaweg 80, 3015 CN Rotterdam, The Netherlands; e-mail: j.cornelissen@erasmusmc.nl.
References
Author notes
The full-text version of this article contains a data supplement