Key Points
Increasing donor age is associated with higher incidence of aGVHD and NRM and lower incidence of disease relapse after haplo-SCT with PT-Cy.
Parent donors, particularly mothers, are associated with worse PFS and GVHD-relapse-free survival.
Abstract
Donor selection contributes to improve clinical outcomes of T-cell–replete haploidentical stem cell transplantation (haplo-SCT) with posttransplant cyclophosphamide (PT-Cy). The impact of donor age and other non-HLA donor characteristics remains a matter of debate. We performed a multicenter retrospective analysis on 990 haplo-SCTs with PT-Cy. By multivariable analysis, after adjusting for donor/recipient kinship, increasing donor age and peripheral blood stem cell graft were associated with a higher risk of grade 2 to 4 acute graft-versus-host-disease (aGVHD), whereas 2-year cumulative incidence of moderate-to-severe chronic GVHD was higher for transplants from female donors into male recipients and after myeloablative conditioning. Increasing donor age was associated with a trend for higher nonrelapse mortality (NRM) (hazard ratio [HR], 1.05; P = .057) but with a significant reduced risk of disease relapse (HR, 0.92; P = .001) and improved progression-free survival (PFS) (HR, 0.97; P = .036). Increasing recipient age was a predictor of worse overall survival (OS). Risk of relapse was higher (HR, 1.39; P < .001) in patients aged ≤40 years receiving a transplant from a parent as compared with a sibling. Moreover, OS and PFS were lower when the donor was the mother rather than the father. Pretransplant active disease status was an invariably independent predictor of worse clinical outcomes, while recipient positive cytomegalovirus serostatus and hematopoietic cell transplant comorbidity index >3 were associated with worse OS and PFS. Our results suggest that younger donors may reduce the incidence of aGVHD and NRM, though at higher risk of relapse. A parent donor, particularly the mother, is not recommended in recipients ≤40 years.
Introduction
Haploidentical stem cell transplantation (haplo-SCT) has become an increasingly used approach after the introduction of high-dose posttransplant cyclophosphamide (PT-Cy) that avoids T-cell depletion of the graft.1 Haplo-SCT with PT-Cy is widely considered an acceptable alternative strategy when a matched related donor (MRD) or unrelated donor (UD) is not available. This is supported by registry- and single-center–based retrospective studies showing comparable outcomes among haploidentical, MRD, or UD allogeneic SCT both for patients with myeloid malignancies2,3 or lymphomas.4,5
Given the broad availability of potential haploidentical family members, selection of the best-available donor may contribute to improve clinical outcomes of haplo-SCT with PT-Cy. Unlike the setting of matched UD transplantation where HLA mismatches were associated with worse outcome, “haploidentical” HLA disparity between donors and recipients did not result in differences in event-free survival and graft-versus-host disease (GVHD) incidence.6 Therefore, non-HLA donor characteristics were analyzed in several reports that resulted in a recent consensus for the selection of an optimal haplodonor for the PT-Cy platform from the European Society for Blood and Marrow Transplantation (EBMT).7 While a selection algorithm incorporating the presence of donor-specific antibodies (DSAs),8 ABO incompatibility,9 and cytomegalovirus (CMV) serostatus10 is accepted by most centers,11 other donor/recipient characteristics are still a matter of debate. In particular, conflicting results were published on the role of donor age. On one hand, some retrospective studies have shown that recipient and disease characteristics are more important than donor characteristics on transplant outcomes.12-15 On the other hand, increasing donor age was found to significantly affect transplant outcomes of older recipients, either when they were aged ≥40 years or when myelodysplastic syndromes were their primary diseases.16,17 Similarly, the usage of a parent donor was associated with an increased risk of graft failure and lower survival in some studies,13 but this effect may change based on recipient age.14 In addition, another report16 has shown an adverse impact of sibling and children donors on transplant outcome, but this effect was dependent on donor and recipient age.
We therefore investigated the potential impact of non-HLA donor characteristics, in particular donor age, in a multicenter retrospective cohort of 990 patients treated with haplo-SCT and PT-Cy. We also analyzed whether the effect of donor age was independent by recipient age and donor/recipient kinship.
Patients and methods
This is a retrospective, observational, cohort study of 990 patients with hematological malignancies treated with haplo-SCT with PT-Cy at 8 transplant centers in Italy (Istituto Clinico Humanitas, Milan; Policlinico San Martino, Genoa; Ospedale Molinette, Turin; Carlo Melzi Unit, Udine; Ospedale Bianchi-Melacrino-Morello, Reggio Calabria; Federico II University, Naples; and Fondazione Casa Sollievo della Sofferenza, San Giovanni Rotondo) and France (Paoli-Calmettes Institute, Marseille) between February 2009 and December 2017. The study was approved by the institutional review board of the Humanitas Cancer Institute, coordinating center, and by the participating centers. Patients provided informed consent for the retrospective collection of their clinical data. All procedures were performed in accordance with the Ethical Standards of the Responsible Committee on Human Experimentation (institutional and national) and with the Helsinki Declaration of 1975 as revised in 2008.
Inclusion criteria were as follows: donors represented by first- or second-degree relatives who were identical at 1 HLA haplotype and mismatched at ≥2 loci of the unshared haplotype, recipient age ≥18 years, and high-dose PT-CY as GVHD prophylaxis. A previous allogeneic SCT was an exclusion criterion.
Conditioning regimen and GVHD prophylaxis
The conditioning regimens employed, both myeloablative conditioning (MAC) and reduced-intensity conditioning/nonmyeloablative (RIC/NMA), are summarized in Table 1. A MAC regimen was defined as a conditioning containing either total body irradiation (TBI) with a total dose >8 Gy or a total dose of IV busulfan >6.4 mg/kg body weight. All other regimens were defined as RIC/NMA.18 Most commonly used conditionings included (1) either the association of thiotepa (5 mg/kg × 2), IV busulfan (3.2 mg/kg per day for 3-4 days), and fludarabine (30 mg/m2 per day for 5 days) or a TBI-based regimen with/without cyclophosphamide or fludarabine for MAC; (2) either the “Baltimore” platform (cyclophosphamide 14.5 mg/kg on days −5 and −6, fludarabine 30 mg/m2 from day −6 to day −2, and low-dose TBI [2 Gy] on day −1) or a combination of IV busulfan (3.2 mg/kg per day for 2 days) and fludarabine (30 mg/mq per day for 5 days) with/without thiotepa (5 mg/kg for 1 day) for RIC/NMA.
GVHD prophylaxis consisted of 2 regimens (Table 1): (1) a standard PT-Cy regimen (cyclophosphamide 50 mg/kg administered on days +3 and +4 and tacrolimus [FK] or cyclosporine A [CsA] and mycophenolate mofetil [MMF] from day +5) and (2) a modified PT-Cy regimen (cyclophosphamide 50 mg/kg administered on days +3 and +5, FK or CsA from day 0, and MMF from day +1). FK at a total dose of 1 mg was administered as a continuous infusion during hospitalization and converted into oral formulation after discharge. CsA was given at 3 mg/kg as a continuous infusion or bolus infusion every 12 hours until discharge and then converted into an oral formulation. Dosages were adjusted to maintain serum levels within therapeutic ranges (FK between 5 and 10 ng/mL and CsA between 100 and 300 ng/mL). MMF was administered at 15 mg/kg p.o. 2 or 3 times per day until day +35. FK and CsA were tapered from day +100 through +180 in the absence of GVHD.
Stem cell source and donors
Potential family members were HLA typed at the HLA-A, HLA-B, HLA-DRB1, and HLA-DQB1 loci at a high-resolution level. Selected donors were also typed at the HLA-C locus at a high-resolution level. Donors underwent bone marrow (BM) harvest under general anesthesia or were mobilized by subcutaneous granulocyte colony-stimulating factor at 10 μg/kg/day for 5-6 days. Unmanipulated BM and peripheral blood stem cells (PBSCs) were infused on day 0.
Engraftment and GVHD evaluation
Granulocyte colony-stimulating factor was started on day +5 in all patients. Neutrophil engraftment was defined as the first of 3 consecutive days with an absolute neutrophil count 0.5 × 109/L after transplantation. Platelet engraftment was defined as a steady platelet count ≥20 × 109/L, with no transfusion requirements during the preceding 7 days. Acute GVHD (aGVHD) was graded by the Keystone criteria19 and chronic GVHD (cGVHD) by National Institutes of Health criteria.20
Statistical analysis
Outcomes were defined by the EBMT statistical guidelines, and GVHD-free/relapse-free survival as reported by Holtan et al.21 Observation period started on day 0. Time-to-event outcomes not affected by competitive events (ie, progression-free survival [PFS], OS, GVHD/relapse–free survival [GRFS]) were estimated using the Kaplan-Meier method,22 and differences according to donor and recipient characteristics were evaluated using the stratified Cox proportional hazard model, using donor type as stratification factor. Time-to-event outcomes affected by competitive events (all-cause mortality for cumulative incidence of aGVHD, cGVHD, and relapse incidence; death for relapse and nonrelapse mortality [NRM]) were estimated using the method proposed by Gooley et al.23 and differences were evaluated using the stratified Fine & Gray model,24 using donor/patient relationship as stratification factor. Factors affecting graft failure were investigated using the logistic regression model. Hazard ratios (HRs) and odds ratios were presented along with their corresponding 95% confidence intervals (CIs). Subgroup analyses on cumulative incidence of aGVHD were performed to evaluate a potential heterogeneity of donor-age effect according to recipient characteristics. For each subgroup, a Fine and Gray model was estimated adjusting for all factors, and the presence of the effect modification was tested by including an interaction term between the donor age (as continuous) and the subgroup covariate of interest. In all estimated effects, clustering of patients within centers was accounted for by adjusting the standard errors with the Huber-White sandwich estimator.25 On subgroups of patients defined by the recipient age, an explorative analysis was performed to evaluate the effect of donor kinship on main outcomes. Given the small sample size of each subgroup, the effect of donor kinship was adjusted using a propensity score approach, including the following factors in the propensity score estimate: gender, disease type, pretransplant disease status, CMV serostatus, hematopoietic stem cell transplant comorbidity index (HCT-CI), graft source (BM vs PBSC), and conditioning regimen. All analyses were performed using STATA version 14.0.
Results
Patients, transplant, and donor characteristics
The study population consisted of 990 patients who received a haplo-SCT with PT-Cy. Myeloid malignancies accounted for 59% of all the diagnoses; the most common diagnoses were acute myeloid leukemia (AML) (39%) and myelodysplastic syndrome (10%), while among lymphoid diseases, acute lymphoblastic leukemia, Hodgkin lymphoma and non-Hodgkin lymphoma represented 12%, 11%, and 13%, respectively, of the whole population. Most patients were in complete remission (56%) or had a low-intermediate disease risk index26 before transplantation. Most patients (63%) had a HCT-CI27 <3. A BM graft was the main hematopoietic cell source (62%), and conditioning regimen was NMA for 64% of the patients. Median donor age was 37 years (25% to 75% CI, 28-49); an offspring was most commonly represented (47%), followed by a sibling (35%) and a parent donor (15%).
Hematopoietic recovery
Graft failure occurred in 45 patients (4.5%; primary in 44 patients and secondary in 1 patient). Complete data on DSAs were not available, preventing any correlation between presence of DSAs and graft failure. Median times to neutrophil and platelet recovery were 19 days (25% to 75% CI, 16-23) and 27 days (25% to 75% CI, 21-35), respectively. Hematopoietic recovery was not correlated either with donor age or kinship.
aGVHD and cGVHD
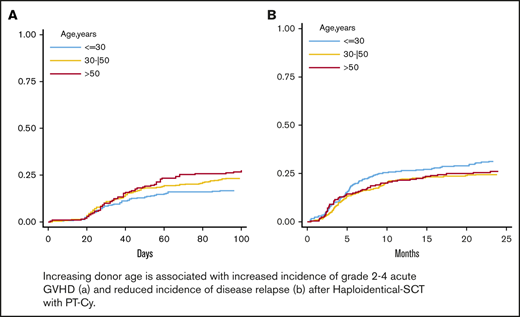
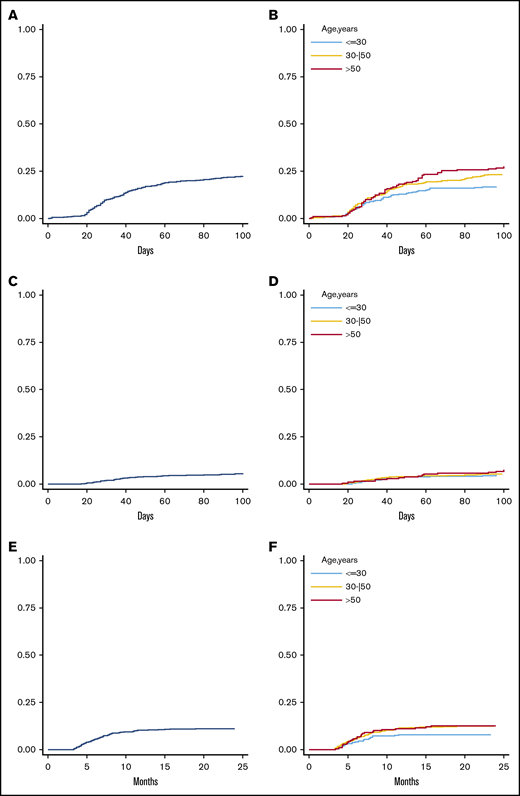
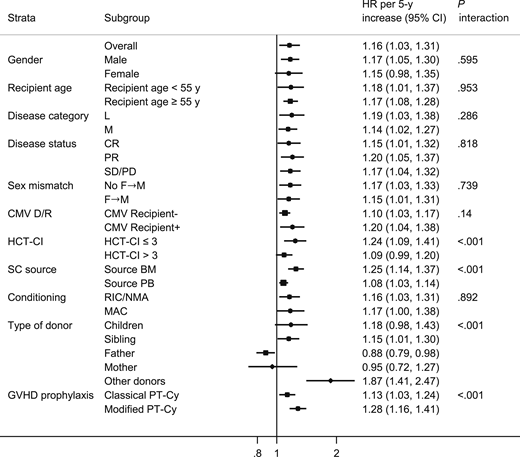
The 100-day cumulative incidence of grade 2 to 4 aGVHD was 22% (Figure 1A) for all patients. By multivariable analysis using donor/recipient kinship as stratification factor (Table 2), donor age (HR; 1.16; P = .015), analyzed by 5-year increments, and PBSC graft source (HR, 1.74; P = .003) were associated with a significantly increased risk of grade 2 to 4 aGVHD. Having a lymphoid rather than a myeloid malignancy (HR, 0.75; P = .020) was a predictor of a higher incidence of grade 2 to 4 aGVHD. A constant increase in the cumulative incidence of grade 2 to 4 aGVHD was observed with increasing donor age; the 100-day cumulative incidence of grade 2 to 4 aGVHD was 17% for donors <30 years, 24% for donors aged 30 to 50 years, and 27% for donors >50 years (Figure 1B).
Cumulative incidence of aGVHD and cGVHD. The 100-day cumulative incidence of grade 2 to 4 aGVHD in the whole population (A) and according to donor age (B) (≤30 years, 30-50 years, and >50 years). The 100-day cumulative incidence of grade 3 to 4 aGVHD in the whole population (C) and according to donor age (D) (≤30 years, 30-50 years, and >50 years). Two-year cumulative incidence of moderate-to-severe cGVHD in the entire population (E) and according to donor age (F) (≤30 years, 30-50 years, and >50 years).
Cumulative incidence of aGVHD and cGVHD. The 100-day cumulative incidence of grade 2 to 4 aGVHD in the whole population (A) and according to donor age (B) (≤30 years, 30-50 years, and >50 years). The 100-day cumulative incidence of grade 3 to 4 aGVHD in the whole population (C) and according to donor age (D) (≤30 years, 30-50 years, and >50 years). Two-year cumulative incidence of moderate-to-severe cGVHD in the entire population (E) and according to donor age (F) (≤30 years, 30-50 years, and >50 years).
Recipient age (either for recipients <55 or ≥55 years; interaction P = .971), pretransplant disease status (either complete or partial remission or stable/progressive disease), disease subtype (lymphoid or myeloid), female→male allograft, conditioning regimen (MAC or RIC/NMA), or recipient CMV serostatus did not significantly affect aGVHD incidence (Table 3). By contrast, a different effect of donor age was correlated with donor/recipient kinship. Increasing donor age was associated with a higher occurrence of aGVHD when the donor was a sibling, an offspring, or a collateral (Table 3), but it was not a risk factor in case of a mother donor, and it was somewhat protective against aGVHD in case of a father donor. Of note, donor age had a significant impact on grade 2 to 4 aGVHD regardless of the PT-Cy prophylaxis employed, though it was more pronounced (without reaching statistical significance) when the modified Pt-Cy regimen was used, in cases of BM grafts rather than PBSC grafts, and in recipients with an HCT-CI <3 rather than ≥3 (Table 3).
The 100-day cumulative incidence of grade 3 to 4 aGVHD was 6% (Figure 1C) for the whole population. By multivariable analysis, a PBSC graft (HR: 1.86, p≤.001), a MAC regimen (HR: 2.53, P < .001) and a HCT-CI ≥ 3 (HR: 1.55, P = .015) were independent predictors of increased risk of grade 3 to 4 aGVHD (Table 2). Increasing donor age was not an independent variable for the whole population (Table 2 and Figure 1D) but maintained a significant impact on grade 3 to 4 aGVHD incidence when the recipient was ≥55 years (HR, 1.22; 95% CI, 1.06-1.41) and in cases of lymphoid malignancy (HR, 1.20; P interaction = .021) (supplemental Table 1). Moreover, the PT-Cy regimen did not affect the impact of age on the risk of grade 3 to 4 aGVHD (supplemental Table 1)
The 2-year cumulative incidence of moderate-to-severe cGVHD was 11% for the entire population (Figure 1E). By multivariable analysis, female donor→male recipient (HR, 2.58; P < .001), recipient CMV-positive serostatus (HR, 1.89; P < .001), and a MAC regimen (HR, 1.81; P = .027) were associated with an increased risk of moderate-to-severe cGVHD (Table 2). Increasing donor age was not an independent predictor of moderate-to-severe cGVHD, but a trend for increased moderate-to-severe cGVHD was observed when the donor was ≥30 years old (2-year cumulative incidence of moderate-to-severe cGVHD was 8% for donors <30 years, 12% for donors aged 30-50 years, and 13% for donors >50 years) (Figure 1F). Of note, age had a similar impact on the risk of moderate-to-severe cGVHD regardless of the PT-Cy regimen employed (supplemental Table 2).
NRM and relapse
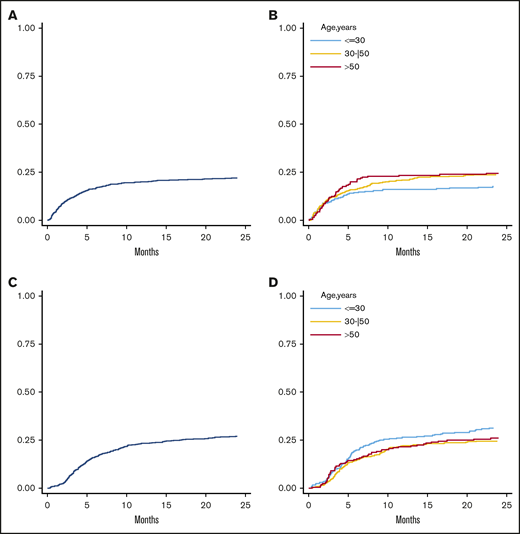
Overall, 228 (23%) patients died of NRM, and the rate was 23% for all patients (Figure 2A). Main causes of death were infections (n = 99), GVHD (n = 31), veno-occlusive disease/transplant-related microangiopathy (n = 10), central nervous system toxicity (n = 10), graft failure/poor graft function (n = 7), and cardiac toxicity (n = 7) (supplemental Table 3). By multivariable analysis, increasing recipient age by 5-year increments (HR, 1.010; P = .004), active pretransplant disease status (HR, 1.61; P < .001), and recipient CMV-positive serostatus (HR, 1.74; P = .042) were independent predictors of increased NRM (Table 4). There was a trend toward a higher rate of NRM with increasing donor age (HR, 1.05), though not reaching statistical significance (P = .057). A continuous increase of NRM with older donor age was indeed observed; 1-year NRM was 16% for donors <30 years, 21% for donors aged 30 to 50 years, and 23% for donors >50 years (Figure 2B). Similar to what was observed for aGVHD, the detrimental effect of donor age on NRM was independent of recipient age (P interaction = .554), pretransplant disease status, and HCT-CI (supplemental Table 4). Increasing donor age was also associated with a higher NRM incidence in case of sibling or offspring donors but was protective when the donor was the father (P interaction < .01; supplemental Table 4).
Cumulative incidence of NRM and disease relapse. Three-year NRM in the whole population (A) and stratified by donor age (B) (30 years, 30-50 years, and >50 years). Three-year cumulative incidence of disease relapse in the entire population (C) and according to donor age (D) (≤30 years, 30-50 years, and >50 years).
Cumulative incidence of NRM and disease relapse. Three-year NRM in the whole population (A) and stratified by donor age (B) (30 years, 30-50 years, and >50 years). Three-year cumulative incidence of disease relapse in the entire population (C) and according to donor age (D) (≤30 years, 30-50 years, and >50 years).
At a median follow-up of 41.4 months for alive patients, 223 experienced disease relapse at a median of 160 days posttransplant. Overall, 3-year cumulative incidence was 29% (Figure 2C). By multivariable analysis (Table 4), increasing donor age (HR: 0.92, P = .001) and pretransplant complete remission as compared with progressive disease (HR: 2.50, P < .001) were significantly associated with reduced risk of relapse. The protective effect of increasing donor age was independent of recipient age (HR, 0.90 for recipient <55 years and 0.92 for recipients ≥55 years; P = .673), pretransplant disease status, and disease subtype. However, as for GVHD and NRM incidence, it was offset when the donor was the mother (HR, 1.10; P interaction < .01; supplemental Table 5) and confirmed with all other donors. Moreover, the effect of donor age on disease relapse was more evident when the donor was >30 years (Figure 2D).
OS, PFS, and GRFS
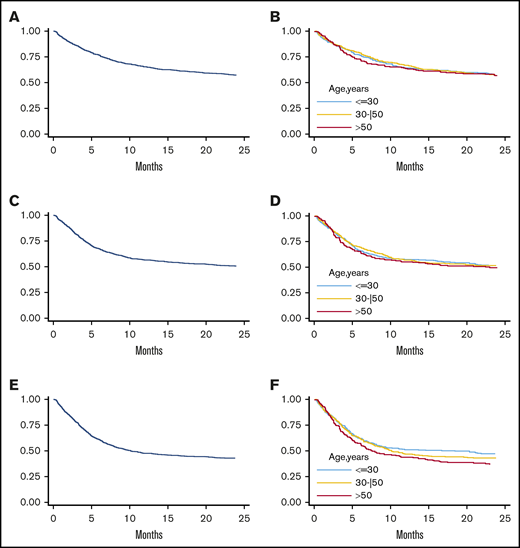
Overall, 3-year OS, PFS, and GRFS rates were 55% (Figure 3A), 48% (Figure 3C), and 41% (Figure 3E), respectively. By multivariable analysis, pretransplant active disease (HR, 2.57; P < .001), increasing recipient age (HR, 1.03; P = .001), HCT-CI ≥3 (HR, 1.34; P = .002), and a recipient CMV-positive serostatus (HR, 1.46; P < .001) were independent variables associated with worse OS (Table 4). Consistent with a reduced risk of relapse, donor age had a protective effect for PFS (HR, 0.97; P = .036), while it did not significantly affect OS (Table 4 and Figure 3B). Pretransplant active disease status, HCT-CI ≥3, and recipient CMV-positive serostatus were other independent predictors of worse PFS and GRFS (Table 4). Of note, increasing donor age was not an independent predictor for GRFS (HR, 1.00; P = .974), though a constant decrease of 36-month GRFS was observed (44% for donors <30 years, 41% for donors aged 30-50 years, and 37% for donors >50 years) (Figure 3F).
Analysis of clinical outcome in terms of OS, PFS, and GRFS. Three-year OS in the whole population (A) and stratified by donor age (B). Three-year PFS in the whole population (C) and according to donor age (D). Three-year GRFS in the entire population (E) and stratified according to donor age (F) (≤30 years, 30-50 years, and >50 years).
Analysis of clinical outcome in terms of OS, PFS, and GRFS. Three-year OS in the whole population (A) and stratified by donor age (B). Three-year PFS in the whole population (C) and according to donor age (D). Three-year GRFS in the entire population (E) and stratified according to donor age (F) (≤30 years, 30-50 years, and >50 years).
Effect of donor/recipient relationship on transplantation outcome
Given that the donor-aging effect on main outcomes was consistent in children and siblings but differed from parents, we addressed whether a certain kinship was associated with a better outcome, particularly whether a father or mother as a donor may play a role in the donor choice algorithm. Donor age strongly correlated with patient age, as well as with donor age and donor/recipient kinship. Therefore, to address the effect of donor/recipient relationship we split our cohort in recipients ≤40 years of age, where the donor was mainly represented by a parent or a sibling, and >40 years, where donors were mainly siblings and offspring. While we did not detect any difference in outcomes after transplantation in grafts from a sibling or a child in recipients >40 years (supplemental Tables 7-9), a parent graft, from either a mother or father, was associated with a higher incidence of disease relapse (HR, 1.39; P < .001) and a worse PFS (HR, 1.32; P < .001) and GRFS (HR, 1.34; P < .001) relative to a graft from a sibling in recipients ≤40 years (Table 5). The dismal PFS was mainly due to a higher incidence of cGVHD (HR, 2.46; P = .017) with a father donor and a higher incidence of disease relapse (HR, 1.64; P = .001) with a mother donor.
A mother graft had a similar incidence of aGVHD and cGVHD relative to a transplant from a father. Interestingly, a transplant from a mother was associated with a worse OS (HR, 2.29; P < .01), PFS (HR, 1.78; P = .01), and GRFS (HR, 1.75; P = .026) and a trend for increased NRM (HR, 3.126; P = .43) relative to a father graft (supplemental Table 6).
Discussion
This retrospective study confirms and extends previous findings concerning the relevance of optimizing donor selection to improve clinical outcomes of T-cell–replete haplo-SCT with PT-Cy. We found that donor age was associated with increased incidence of aGVHD and reduced occurrence of disease relapse, resulting in increased NRM and improved PFS rates. Moreover, we observed that the effect of donor age was independent of recipient age, though not constant across all subtypes of donor/recipient kinship, particularly in cases of parent donors.
The effect of donor age was initially investigated in unrelated donor SCT. Kollman et al reported a 5.5% increase in HR for mortality28 and an 8% increase for aGVHD and cGVHD incidence for every 10-year increment in donor age.29 Rezvani et al,30 however, showed similar GVHD and NRM rates between donors <60 and ≥60 years in related-donor SCT. Both this study29 and another one from the Center for International Blood and Marrow Transplant Research31 reported a worse outcome in younger recipients of matched unrelated donor SCT as compared with older recipients of marrow related transplant. However, both reports refer to years when HLA matching was not based on high HLA resolution methods. In the setting of T-cell–replete Haplo-SCT, recently, increased attention has been drawn to non-HLA donor characteristics. In the GIAC (G-CSF–priming of the donor, intensified immunosuppression, ATG, and combination of T-cell–replete BM plus peripheral blood as the stem cell source) platform, the Beijing group described that transplants from younger male donors were correlated with lower NRM and better OS rates.32 Conflicting results have been reported with the PT-Cy platform. Canaani et al,16 in a retrospective analysis of 1270 AML patients, found that increasing donor age significantly affected NRM and leukemia-free survival in patients >40 years, but not younger ones. Consistently, Ciurea et al17 found a negative effect of older donor age (>40 years) on OS in a small cohort of patients ≥55 years. Overall, differences in recipient and donor age cutoffs, proposed by the various studies, have not yet allowed us to draw definitive conclusions that impact daily clinical practice. In this report, we have shown for the first time that older donor age has a negative impact on aGVHD and NRM and that this effect is continuous as donor age increases without the need to set age cutoffs. Of note, this effect is almost uniformly independent of recipient age. Our findings may differ from those reported by Canaani et al,16 as our analysis included patients with both myeloid and lymphoid malignancies, whereas the EBMT study focused on AML patients only. As a matter of fact, in our analysis, patients with a myeloid malignancy showed a lower risk of grade 2 to 4 aGVHD than those with a lymphoid malignancy. Other large retrospective studies12-15 have provided conflicting results, showing that patient and disease characteristics, rather than donor age, are the main predictors of OS, PFS, and NRM. However, consistent with previous findings, we also confirm that recipient age, HCT-CI, and pretransplant disease status remain important variables capable of affecting OS, PFS, and NRM. Different findings among studies may likely be due to their retrospective design and potential selection bias. Nevertheless, the fact that our multicenter analysis is not registry based may have allowed for a more detailed and accurate collection of events such as GVHD and NRM.
A biological explanation that reliably accounts for the effect of donor age on aGVHD is currently elusive. Chen et al33 also described an increased incidence of grade 2 to 4 aGVHD with older donor age after haplo-SCT. In their platform that included CD34+ selected graft and donor lymphocyte infusion followed by PT-Cy, the authors were able to accurately monitor the effect of donor lymphocyte infusion composition on outcomes. They observed that increased CD4 content and CD4/CD8 ratio from donors between the ages of 46 and 55 years were associated with increased incidence of aGVHD. It may also be speculated that T memory stem cells, which we previously reported to be the most abundant circulating T-cell population in the early days following haplo-SCT with PT-Cy34 and were shown to be associated with increased incidence of GVHD in preclinical models,35 may be more prominent in older donors. Preclinical models have also shown that aging is associated with a reduced number of inducible T regulatory cells, while naive T regulatory cells increase.36,37 It may be postulated that a different inducible T regulatory/naive T regulatory cell ratio in older donors may affect incidence of aGVHD. Moreover, impaired thymic function in aging donors may contribute to different composition of the graft and impact on the risk of GVHD. Further biological studies, focused on CD4 T-cell graft content and on posttransplant T memory stem cell fate, are warranted to potentially correlate GVHD and donor age.
A novel finding of our report is the association between increasing donor age and reduced risk of disease relapse. This finding was also independent of disease subtype (myeloid vs lymphoid) and pretransplant disease status. Future analyses are needed to investigate whether this effect is more prominent in particular subsets of malignancies (ie, some lymphoma subtypes vs AML vs myelodysplastic syndromes) and find plausible biological evidence. A potential explanation for this observation is that increased incidence of aGVHD with donor aging may be associated with an enhanced immune-mediated graft-versus-tumor effect. The potential beneficial effect of aGVHD on disease relapse was first shown in the HLA-identical setting38 and more recently confirmed by the Johns Hopkins group, who associated grade 2 aGVHD with reduced relapse rate and enhanced PFS in patients receiving PT-Cy after either a haploidentical39 or HLA-matched transplant.40 Of note, in our analysis, grade 2 aGVHD partly contributed to a reduced risk of relapse, without reaching statistical significance (HR, 0.76; P = .237; data not shown). Thus, a younger donor may be preferable to reduce the risk of aGVHD and NRM, as in the setting of nonhematologic malignancies or for very frail patients, whereas an older donor may represent a better choice in high-risk diseases requiring a stronger graft-versus-tumor effect.
We also observed that the effect of donor age was independent of donor/recipient kinship, with the exception of parent donors, where differences between mothers and fathers were observed. Recent publications suggest that a parent donor may be associated with a worse outcome because of either an increased incidence of graft failure for older donors when recipients were <55 years14 or an increased incidence of disease relapse resulting in poorer OS and PFS.3 Even though our study was primarily focused on the effect of donor age, we also evaluated the potential role of kinship in donor selection. With the limits of the sample size, we confirmed that a parent donor is associated with a worse clinical outcome as compared with a sibling in patients ≤40 years. Moreover, our observation that the mother donor is worse than the father is somewhat consistent with findings from the Beijing group with the GIAC platform, where mother donors were associated with worse NRM and GVHD rates.32 On the contrary, our observations are not consistent with previous reports by van Rood et al41 and Ichihohe et al,42 which described in a non–T-cell-depleted setting a reduced incidence of GVHD and an improved outcome when the donor was the mother or a noninherited maternal antigen–mismatched sibling. These differences may be due to the different GVHD prophylaxes (non–PT-Cy based) and conditionings employed. Overall, our results should be interpreted with a degree of caution, as parent donors represented only 15% of our donors. Due to the small number of transplants from collateral donors (n = 32), we could not carry out a fair comparison between collateral and first-degree relatives. Among other donor characteristics associated with a worse outcome, we confirmed a role for female→male allograft6,32 due to donor immunity to minor histocompatibility antigen–encoded genes on the Y chromosome.43,44
We have also shown that recipient CMV-positive serostatus independent of donor CMV serostatus was associated with a worse outcome in terms of OS, PFS, NRM, cGVHD, and GRFS. These results are consistent with the finding by Kasamon et al6 and Cesaro et al45 that did not document any effect of the donor CMV serostatus on haplo-SCT outcome. Therefore, our results are consistent with the recent EBMT guidelines that recommend choosing a donor regardless of the CMV serostatus.7
Briefly, among nondonor characteristics, while the role of pretransplant disease status12 and HCT-CI46 in transplant outcomes is widely recognized, the impact of graft source, either BM or PBSC, remains hotly debated. In our cohort, the use of PBSC was the most significant variable associated with increased risk of grade 2 to 4 and 3 to 4 aGVHD, consistent with other recent retrospective reports14,47,48 from both the EBMT and the Center for International Blood and Marrow Transplant Research. By contrast, we did not confirm a significant association between PBSCs and the incidence of cGVHD. This may be due to either selection bias or differences in the analyses of these retrospective studies. For instance, in our analysis, only moderate-to-severe cGVHD was investigated to reduce possible “operator-dependent” bias of GVHD grading of mild forms. Of note, no significant impact was associated with either of the 2 PT-Cy regimens employed in this study, which is consistent with a recent report.49 Finally, future prospective studies should include, among other variables, detailed HLA and killer cell immunoglobulin-like receptor typing for all donor/recipient pairs, and presence/absence of DSA to evaluate their impact on disease relapse,13 graft failure50 and the correlation between non-inherited maternal antigen7,32 and clinical outcomes.
In conclusion, though retrospective, this is one of the largest analyses on the role of non-HLA donor characteristics on clinical outcomes in haplo-SCT with PT-Cy. Our results suggest that when more haploidentical donors are available, a younger donor may reduce the risk of aGVHD and NRM at the expense of a potentially higher risk of disease relapse. Our data further confirm that a male donor for a male recipient may be the optimal choice, while a sibling or an offspring may be a better choice than a father and then a mother.
Requests for data sharing should be e-mailed to the corresponding author, Luca Castagna (e-mail: castagnal@ipc.unicancer.fr).
Acknowledgments
The authors thank Laura Giordano and Clara Di Vito for critical discussion.
This work was supported by Fondazione Cariplo grant 2015/0603 (D.M. and B.B.), Associazione Italiana per la Ricerca sul Cancro grant IG21567, and intramural research funding from Istituto Clinico Humanitas (5*1000 project) (D.M. and L.C.).
Authorship
Contribution: J.M. performed research, analyzed and interpreted data, and wrote the manuscript; A.M.R. collected data and provided data interpretation; A.E. provided data analysis and interpretation; A.M.C., M.M., F.P., and A.R. collected data and supervised the manuscript; S.B., A.B., L.G., L.B., B.L., G.C., R.F., A. Sperotto, L.M., S.M., C.F., S.S., P.C., E.M., L.S., S.H., and S.F. collected data; A. Santoro, A.B., D.B., and E.A. provided data interpretation and revised the manuscript; and D.M., L.C., and B.B. designed research, provided data interpretation, and supervised manuscript writing.
Conflict-of-interest disclosure: A. Santoro is on the advisory board at Bristol-Myers Squibb, Servier, Gilead, Pfizer, Eisai, Bayer, and Merck Sharp & Dohme; receives consultancy fees from Arqule/Sanofi; and receives speaker’s bureau fees from Takeda, Bristol-Myers Squibb, Roche, AbbVie, Amgen, Celgene, Servier, Gilead, AstraZeneca, Pfizer, Arqule, Lilly, Sandoz, Eisai, Novartis, Bayer, and Merck Sharp & Dohme. The remaining authors declare no competing financial interests.
Correspondence: Luca Castagna, Humanitas Research Hospital, IRCCS, Via Manzoni 56, Rozzano, 20080, Italy; e-mail: luca.castagna@cancercenter.humanitas.it.
References
Author notes
J.M. and A.M.R. contributed equally to this study.
D.M., L.C., and B.B. contributed equally to this study.
The full-text version of this article contains a data supplement.