Key Points
Therapy with an IL-15 superagonist resulted in immune and clinical responses in a transplant recipient with PML.
Introduction
Progressive multifocal leukoencephalopathy (PML) is a rare demyelinating disease of the central nervous system caused by reactivation of the John Cunningham virus (JCV). JCV is common; however, most infected patients will remain asymptomatic.1 In the setting of immunosuppression, including after allogeneic hematopoietic cell transplant (HCT), the virus is transformed and may infect oligodendrocytes, causing a course of progressive neurologic decline. PML is generally rapidly fatal. There is no effective antiviral treatment of JCV or PML, but immune reconstitution is essential. In the post-HCT setting, the risk of developing PML does not appear to have an end point, and time to development of symptoms can range from weeks to decades.
Interleukin-15 (IL-15) is critical for the proliferation and activation of natural killer (NK) cells and CD8+ memory T cells. N-803, a novel IL-15 superagonist, contains a mutant form of IL-15 (N72D), in complex with the soluble domain of the IL-15 receptor (IL-15Rα), resulting in a prolonged serum half-life and increased biologic activity compared with wild-type IL-15.2 Here, we report a case of PML following allogeneic HCT in which neurological improvement occurred following treatment with N-803.
Case description
A 27-year-old HIV-negative male with no significant medical history was diagnosed with T-cell acute lymphoblastic leukemia in January 2016. The patient was treated according to Cancer and Leukemia Group B 10403. Cerebrospinal fluid (CSF) cytology was negative at diagnosis and remained negative throughout therapy. Following a 10/10 matched-unrelated donor allogeneic HCT in May 2016, the patient achieved minimal residual disease negativity with complete donor engraftment. All immunosuppression was tapered off by February 2017. CD4 count at this time was 458. The patient was free of any neurologic deficits and was able to return to work full time.
On 14 July 2017, 460 days posttransplant, the patient presented with left shoulder pain, left arm heaviness, and asymmetric left shoulder fullness but no weakness or neurologic deficits. There was no history of injuries or trauma. Cervical spine magnetic resonance imaging (MRI) was unremarkable, and CSF analysis showed no evidence of leukemia. Ten days later, he developed new weakness in the left lower and upper extremities, with loss of fine motor skills in the left hand and diminished deep tendon reflexes in the left upper and lower extremities but no numbness or sensory deficits. A short course of prednisone (2 mg/kg × 7 days) was administered, with no improvement.
Over the next week, the patient’s weakness progressed to paralysis, and he was no longer able to ambulate, requiring a wheelchair. Brain MRI showed a posterior right frontal subcortical white matter lesion, hyperintense on T2/fluid-attenuated inversion recovery (FLAIR) without enhancement or diffuse restriction, concerning for PML. A repeat lumbar puncture was performed, and qualitative polymerase chain reaction (PCR) was positive for JCV, confirming the diagnosis. Mefloquine (250 mg daily × 3 days, then 250 mg weekly) and mirtazapine 30 mg daily were initiated on 7 August 2017. Due to lack of improvement and progression of weakness after 14 days, N-803, an IL-15 superagonist, (6 μg/kg subcutaneously on days 1, 8, 15, and 22 of a 28-day cycle) was added under compassionate use (single-patient IND #136501) from the US Food and Drug Administration on 21 August 2017.
Methods
Flow cytometry
Cryopreserved peripheral blood mononuclear cells (PBMCs) were analyzed by flow cytometry as previously described.3
VP1-specific CD8+ T cells (peptides VP169-ESDSPNRDMLPCY, VP1183-NTEHKAYLDKNKAY, and VP1329-GTEELPGDPDMMRY from New England Peptide, Gardner, MA) were loaded into HLA-A*01 monomers by UV-mediated exchange and multimers were generated using streptavidin-conjugated phycoerythrin or allophycocyanin (Invitrogen, Carlsbad, CA).4 PBMCs were cultured with peptides for 12 days (primary stimulation) or 19 days (secondary stimulation with peptides on day 12), dual stained with tetramers, and analyzed by flow cytometry.
Results and discussion
Repeat brain MRI after 2 N-803 cycles on 16 October 2017 showed a worsening extent of the FLAIR abnormality (Figure 1). However, the patient’s strength was improving, and treatment was continued. Subsequent brain MRIs demonstrated marked improvement in the T2/FLAIR white matter lesions, and the most recent brain MRI, 759 days after initiation of N-803, continued to demonstrate response. Qualitative CSF JCV PCR has remained undetected since December 2017. CSF specimens were sent to the National Institutes of Health (NIH) for ultrasensitive quantitative JCV PCR, and the DNA copy number decreased from 31 copies/mL in December 2017 to 16 copies/mL in February 2018 and 11 copies/mL in April 2018. Identification of the JCV DNA variant as prototype was performed at the NIH using the Multiplex qPCR assay. N-803 was stopped after 8 total cycles, and the patient continues to take mefloquine and mirtazapine. His neurologic deficits continue to improve, and he is now able to ambulate with a cane. His last follow-up examination was April 2020 (+926 days after initiation of N-803 therapy), and he remains stable with slow and steady neurologic improvement and no evidence of graft-versus-host disease or acute myeloid leukemia.
FLAIR MRI images. Axial FLAIR MRI images were obtained before initiation of N-803 (A), after 2 cycles (B) and 8 cycles (C) of N-803, and 759 days after initiation of N-803 (D), demonstrating improvement in the posterior right frontal subcortical white matter lesion.
FLAIR MRI images. Axial FLAIR MRI images were obtained before initiation of N-803 (A), after 2 cycles (B) and 8 cycles (C) of N-803, and 759 days after initiation of N-803 (D), demonstrating improvement in the posterior right frontal subcortical white matter lesion.
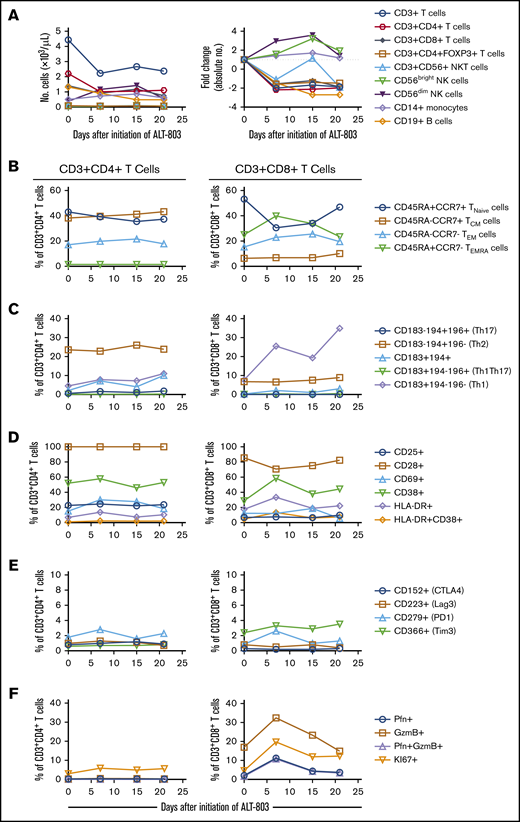
While the absolute number of circulating T and B cells decreased during N-803 treatment, we observed a more than threefold increase in the number of circulating CD56bright and CD56dim NK cells (Figure 2A). Phenotyping of peripheral blood T cell subsets revealed little to no changes within the CD3+CD4+ T cell subsets (Figure 2B-F). In contrast, there was a decrease in the relative percentage of naive CD3+CD8+ T cells with a concomitant increase in effector memory and effector memory RA cells during N-803 treatment. This shift in the CD3+CD8+ T cell population toward an effector phenotype was accompanied by an increase in the number of CXCR3-expressing cells as well as evidence of cell activation as determined by increased expression of CD38, HLA-DR, perforin, granzyme B, and the proliferation marker Ki-67 (Figure 2B-F). These findings are consistent with previously reported data.5,6
Impact of N-803 on peripheral blood cell subsets. Cryopreserved PBMCs were assessed by flow cytometry immediately before (day 0) and at the indicated timepoints after initiation of N-803 treatment. (A) The absolute numbers of cells per microliter of peripheral blood for each subset was determined by multiplying the percentage of a given cell type (relative to the total CD45+ population) by the overall white blood cell count. Fold changes in each subset are relative to the baseline (day 0) sample. (B) Relative percentages of naive, central memory (CM), effector memory (EM), and CD45RA-expressing effector memory (EMRA) CD3+CD4+ or CD3+CD8+ T cell subsets. (C) Relative percentages of CD3+CD4+ or CD3+CD8+ T-cell subsets expressing the chemokine receptors CD183 (CXCR3), CD194 (CCR4), and/or CD196 (CCR6). The Th1, Th2, and Th17 designated in parentheses are only applicable to the CD3+CD4+ T cell subset. (D) The percentage of CD3+CD4+ or CD3+CD8+ T cells expressing cell-surface antigens that are altered during T cell activation. (E) The percentage of CD3+CD4+ or CD3+CD8+ T cells expressing molecules involved in immune checkpoint regulation. (F) The percentage of CD3+CD4+ or CD3+CD8+ T cells expressing intracellular perforin (Pfn), granzyme B (GzmB) or the proliferation marker KI67.
Impact of N-803 on peripheral blood cell subsets. Cryopreserved PBMCs were assessed by flow cytometry immediately before (day 0) and at the indicated timepoints after initiation of N-803 treatment. (A) The absolute numbers of cells per microliter of peripheral blood for each subset was determined by multiplying the percentage of a given cell type (relative to the total CD45+ population) by the overall white blood cell count. Fold changes in each subset are relative to the baseline (day 0) sample. (B) Relative percentages of naive, central memory (CM), effector memory (EM), and CD45RA-expressing effector memory (EMRA) CD3+CD4+ or CD3+CD8+ T cell subsets. (C) Relative percentages of CD3+CD4+ or CD3+CD8+ T-cell subsets expressing the chemokine receptors CD183 (CXCR3), CD194 (CCR4), and/or CD196 (CCR6). The Th1, Th2, and Th17 designated in parentheses are only applicable to the CD3+CD4+ T cell subset. (D) The percentage of CD3+CD4+ or CD3+CD8+ T cells expressing cell-surface antigens that are altered during T cell activation. (E) The percentage of CD3+CD4+ or CD3+CD8+ T cells expressing molecules involved in immune checkpoint regulation. (F) The percentage of CD3+CD4+ or CD3+CD8+ T cells expressing intracellular perforin (Pfn), granzyme B (GzmB) or the proliferation marker KI67.
Using the Immune Epitope Database major histocompatibility complex class I peptide-binding prediction algorithm, we found 3 putative antigens from the JCV major capsid protein VP1 with predicted binding (50% inhibitory concentration <500 nM) to the patient’s HLA-A*01:01 allele. We stimulated PBMCs obtained before and after N-803 treatment with the VP1-derived peptides and stained cultures with peptide-specific HLA-A*01:01 tetramers. No JCV VP1 peptide-specific CD8+ T cells were detected (data not shown).
This report further suggests that N-803 may enhance the clearance and resolution of a viral illness in humans. A preclinical study in monkeys showed that N-803 leads to suppressed replication of an HIV-like virus.7 Preliminary data in HIV-positive patients demonstrated increased numbers of CD4+ and CD8+ T cells, increased NK cells, and increased HIV transcription following N-803.8 In the post-HCT setting, N-803 administration significantly increased NK and CD8+ T cells numbers and function. This phenotype was associated with temporary cytomegalovirus clearance9 and may enhance the clearance of relapsed/refractory acute myeloid leukemia, without increasing rates of immunosuppression-requiring graft-versus-host disease.5 Thus, N-803 may augment antitumor and antiviral immunity in humans.
Novel therapeutic approaches for PML have included immunotherapy, and case reports supporting the use of IL-2,10-13 IL-7,14-20 vaccination,20,21 PD-1 inhibitors,22-26 and adoptive T cell therapy27,28 have been published. Lymphopenia is the main risk factor for PML, and the lesions typically lack a lymphocytic infiltrate. Upon immune restoration, CD8+ T-cell–predominant lymphocytic infiltrates develop, often corresponding with transiently worsening symptoms, MRI findings, or both.29 This process, called immune reconstitution inflammatory syndrome, 30 is perhaps consistent with our patient’s observed MRI findings after 2 cycles of N-803. Brain biopsy/autopsy samples from HIV-positive and HIV-negative patients with PML demonstrated that recruitment of CD8+ T cells is associated with control of JCV infection.31,32 Although HLA-A*02:01-restricted epitopes derived from the JCV VP1-protein have been characterized in PML patients,33-35 we observed no JCP VP1-specific CD8+ T cells to 3 putative peptide epitopes predicted to bind HLA-A*01:01 with high affinity. Our failure to detect JC-specific CD8+ T cells to these 3 peptides suggests that the VP1-derived epitopes determined bioinformatically were either not antigenic or the frequency of responding VP1-specific CD8+ T cells in the peripheral blood was less than the limit of detection (0.1%) of the tetramer-based flow cytometry assay. We acknowledge that our peptide screen for JCV-specific T cells in this patient was very limited and that the JC virus–specific T cells may have been enriched in the CSF rather than the peripheral blood. Although several immunogenic JCV-derived peptides recognized by both CD4+ and CD8+ T cells from PML patients have been reported, none have been restricted to our patient’s HLA-A01*01 allele.27,33-37 Therefore, additional functional studies are needed to determine if N-803 promotes anti-JC virus immunity via NK cells, CD8+ and CD4+ T lymphocytes, or some sort of their combination.
In summary, N-803 represents a possible therapeutic option for treatment of PML in post-allogeneic HCT patients, and further prospective studies are warranted.
For data sharing, e-mail the corresponding author, John F. DiPersio (jdipersi@wustl.edu).
Acknowledgments
The authors thank the patient, his family, and the drug manufacturer (NantCell, LLC, a NantWorks company, Culver City, CA). They also thank Caroline Ryschkewitsch for help with JCV testing; the Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital in St. Louis, MO, for the use of the Siteman Flow Cytometry, which provided flow cytometry access and expertise; and the Immunomonitoring Laboratory, which provided tetramer construction service.
This work was supported by a NIH National Cancer Institute (NCI) Outstanding Investigator Award (R35 CA210084) (J.F.D.), NCI Research Specialist Awards (R50 CA211466 [M.P.R.] and R50 CA211782 [C.A.M.]), and an NCI grant (R01 CA171963) (T.A.F.). The Siteman Cancer Center is supported in part by an NCI Cancer Center Support Grant (P30 CA091842).
Authorship
Contribution: A.O. and M.P.R. contributed equally to this work and were involved in data analysis/interpretation and drafting and editing of the manuscript; D.B.C. was involved in conceptualization; M.P.R., J.R., L.G., J.H., K.O. and P.P. were involved in data analysis and interpretation; E.M. performed the quantitative JCV PCR at the NIH; C.A.M. performed the JCV VP1 HLA-A*01:01 epitope prediction; T.A.F., P.S.-S., and A.R. were involved in editing of the manuscript; and J.F.D. was involved in conceptualization of the study, acquisition of the study drug, analysis/interpretation of the data, and editing of the manuscript.
Conflict-of-interest disclosure: J.F.D. is founder and advisor for Magenta Therapeutics and WUGEN; receives income from Rivervest Venture Partners and Magenta Therapeutics; receives and/or received during the study period research funding from Amphivena Therapeutics, NeoimmuneTech, Macrogenics, Incyte, BiolineRx, Altiris, and WUGEN; and is an advisory board member for Cellworks Group, RiverVest Venture Partners, and Arch Oncology. M.P.R. serves as a consultant for RiverVest Venture Partners, receives research funding from Amphivena Therapeutics, and received research funding from Novimmmune and Cantex during the study. P.S.-S. is an employee of ImmunityBio and is a majority owner of the company. The remaining authors declare no competing financial interests.
Correspondence: John F. DiPersio, Washington University School of Medicine, 660 S Euclid Ave, Box 8007, St. Louis, MO 63110; e-mail: jdipersi@wustl.edu.
References
Author notes
A.O. and M.P.R. contributed equally to this study.